Most members of the marine gastropod family Olividae including the iconic olive shells of the genus Oliva live as predators and scavengers on soft sediments, mostly in the tropics and subtropics (Tursch & Greifeneder 2001). Oliva species forage actively and grab prey items with the anterior part of their foot before they secure them in a pouch formed by the posterior part of the metapodium (Marcus & Marcus 1959, Olsson & Crovo 1968, Taylor & Glover 2000, Kantor & Tursch 2001). Species of the morphologically similar genus Agaronia exhibit specific adaptations to life on sandy beaches such as tidal migrations by underwater sailing locomotion (Peters 2022b), but show the same prey capture behaviour as Oliva (Rupert & Peters 2011). This predatory behaviour is more easily observed in Agaronia species whose foraging habitat, the sandy beach intertidal, is more accessible than that of the mostly subtidal Oliva.
Invertebrate communities on sandy beaches of the central American west coast are a convenient model system for studying predator-prey interactions in the wild. Here we focus on the predatory behaviour of an Agaronia species tentatively identified as A. propatula (Conrad, 1849) (Rupert & Peters 2011). Agaronia propatula relies on its olfactory and mechanical senses to detect and identify potential prey (Cyrus et al. 2012). Since these senses provide only short-range information about a zone of a few cm in front of the animal’s propodium, A. propatula has to search for food by rapidly crawling across the sediment surface in apparently random directions. At our study site in Playa Grande, Costa Rica (10°20'N, 85°51'W), the dominant prey of A. propatula is Pachyoliva semistriata (Gray, 1839; formerly Olivella semistriata [Pastorino & Peters 2023]) of the same family (Robinson & Peters 2018). Interactions between the two are highly dynamic, involving uncommon modes of rapid locomotion (Veelenturf & Peters 2020; see Peters 2022a for a video summary of frequently observed predator-prey behaviour). Agaronia propatula also is cannibalistic and will attack conspecifics in the same manner (Cyrus et al. 2015).
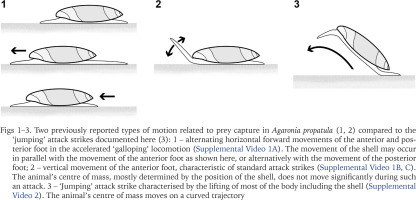
When motivated, for example by encountering a fresh track of Pachyoliva, Agaronia has been shown to switch from standard crawling to a stepping mode of locomotion termed ‘galloping’ (Cyrus et al. 2012). Galloping snails move the anterior and posterior parts of their foot alternatingly without lifting any parts of the sole off the ground (Supplemental Video 1A; Fig. 1). The rapid forward thrust of the anterior foot may result in direct contact with a prey item, but directed attack movements carried out by the anterior foot generally differ from galloping-related movements as they include a more or less pronounced lifting of the foot (Supplemental Video 1B, and Fig. 2; see also Veelenturf & Peters 2020). Such attack strikes may directly follow a galloping-like movement of the anterior foot in which the prey was contacted and thus localised (Supplemental Video 1B). Alternatively, attack strikes are executed without prior galloping, for example when a previously immobile prey betrays its position by starting to move (Supplemental Video 1C).
Figs 1–3
Two previously reported types of motion related to prey capture in Agaronia propatula (1, 2) compared to the ‘jumping’ attack strikes documented here (3): 1 – alternating horizontal forward movements of the anterior and posterior foot in the accelerated ‘galloping’ locomotion (Supplemental Video 1A). The movement of the shell may occur in parallel with the movement of the anterior foot as shown here, or alternatively with the movement of the posterior foot; 2 – vertical movement of the anterior foot, characteristic of standard attack strikes (Supplemental Video 1B, C). The animal’s centre of mass, mostly determined by the position of the shell, does not move significantly during such an attack. 3 – ‘Jumping’ attack strike characterised by the lifting of most of the body including the shell (Supplemental Video 2). The animal’s centre of mass moves on a curved trajectory
It should be emphasised that during a standard attack strike, only part of the foot is lifted while the shell is not (Fig. 2). However, as reported here for the first time, attacking Agaronia occasionally lift the bulk of their body mass including the shell off the ground in a rapid, jump-like motion, in which the prey is struck from above (Fig. 3). While we observe such ‘jumping’ attacks rarely but regularly on the beach, analysing their kinetics and biomechanics is difficult. At this time, we do not know which stimuli or environmental conditions motivate A. propatula to conduct jumping attacks rather than standard attack strikes. Therefore we cannot induce jumping attacks for detailed study, and have to rely on chance observations of spontaneous behaviour. Here we present a jumping attack during a failed cannibalistic predation attempt as a representative example. Coincidentally, the footage documenting the attack (Supplemental Video 2; selected frames are shown as Figs 4–11) visualises the dynamics of the action in a particularly instructive way, because the shadows of the animals provide a second perspective.
Figs 4–11
Phases of a ‘jumping’ attack strike during an unsuccessful cannibalistic predation attempt (compare Supplemental Video 2). Times relative to the first image are given in the lower left of each photograph: 4 – two individuals meet (note the shadows of the attacked (I) and the attacking (II) animals; S marks the shadow of the sipho of animal II); 5 – the attacking animal raises its anterior foot (the shadow of the foot is marked F); 6 – while the anterior foot moves upward, the shell apex tilts downward; 7 – the shell apex touches the sediment (shadow!) while the foot is folded to form a tube (zoom-in on the right); 8 – the anterior edge of the foot reaches its highest elevation (shadow!); 9 – the anterior foot bends forward while the shell apex lifts off the ground; 10 – the animal’s body forms an arc as the propodium hits the target from above; 11 – the jumping attack strike is completed in under 1 s

Immediately after two foraging Agaronia came into contact (Fig. 4), one of the animals began to raise its anterior foot upwards (Fig. 5). In the process, the long axis of the shell turned from a horizontal to an oblique orientation with the apex pointing down (Fig. 6). As the anterior foot kept rising, the shell apex touched the sand and probably functioned like an abutment to facilitate the upward movement of the foot (Fig. 7). In this phase of the movement, the foot had folded lengthwise so that its lateral margins almost touched (Fig. 7). The resulting tube- or pipe-like structure of the foot probably increased the mechanical stability of the upright foot, similarly as a tube such as a plastic straw has a much higher bending stiffness than a plain sheet of the same material and weight. It seems plausible that this feature was essential for the animal to control its movement and direct it to its target. When the foot was maximally expanded in length, the tip of the propodium was about four times as high above the sediment surface as the highest point of the shell was in the crawling animal (Fig. 8; compare shadows in this figure and in Fig. 4). The anterior portion of the foot then bent in the ventral direction (Fig. 9). The shell apex rose from the ground while the animal’s body formed an arc (Fig. 10) as it fell forward to hit the target from above with the propodium (Fig. 11). The entire process took less than a second. In the end, the attack remained unsuccessful, which had to be expected because the two animals were of similar size. Cannibalistic attacks in Agaronia are successful only if the attacker is at least 1.45 times larger than the prey in terms of shell length (Robinson & Peters 2018).
Agaronia’s jumping attack strikes certainly seem impressive, but surprisingly fast movements in gastropods are not unheard of. In most cases, however, for example the rapid motion of the proboscis by which cone snails sting and paralyze small fish (Olivera et al. 2014), it is not the bulk of the body including the shell that moves quickly. Some marine gastropods show rapid locomotion through leaping motions when threatened by predators (e.g., Weber 1924, Gonor 1966, Hoffman 1980). The direction of such leaps usually is more or less random, in contrast to the jumping attacks performed by A. propatula that obviously are directed at specific targets.
It is an interesting question whether jumping attack strikes are possible under water as well, or if this behaviour could only evolve in the context of the adaptation of Agaronia to its semi-terrestrial hunting grounds. Consider, for example, an Agaronia of 30 mm shell length. These animals typically weigh about 2.6 g, of which two thirds are contributed by the shell (own unpublished results). Assuming densities of 1.05 g mL−1 and 2.8 g mL−1 for the soft body and shell, respectively, we estimate that submerged in sea water, the effective weight (i.e., the animal’s mass minus its buoyancy) is reduced by over 40% compared to air. Thus it might seem that ‘jumping’ is easier under water. However, the resistance, or drag that the animal experiences when moving through fluids like air or seawater depends linearly on the fluid’s density (Vogel 1994). The density of seawater is over 800 times that of air, which lets it appear doubtful that Agaronia can ‘jump’ under water at all despite the reduced effective weight. On the other hand, the lower effective weight together with the higher drag would significantly reduce the velocity by which an Agaronia falls down on its prey in the last phase of a jumping attack strike executed under water, increasing a mobile prey’s chance to escape.
We expect that truly quantitative insights into the effects of the physical environment on the evolution of biomechanical and behavioural traits in intertidal gastropods will result from future comparative studies of subtidal Oliva species and the closely related, intertidal Agaronia.

