INTRODUCTION
The genus Amphioctopus P. Fischer, 1882 currently comprises approximately 16 nominal species. However, this number continues to grow as new species are described and cryptic species within species complexes are increasingly identified through integrative taxonomic approaches (e.g., Huffard & Hochberg 2005, Guerrero-Kommritz & Rodrigues-Bermudez 2019, Tang et al. 2020, Xu et al. 2022). Among the species of the genus, the brown-striped octopus A. burryi Voss, 1950 is the only non-ocellated species present in Atlantic waters. However, other species may exist, as the status of several historically described Atlantic species remains unresolved (Norman & Hochberg 2005). These include Octopus rugosus (Bosc, 1792) from Senegal, O. granulatus Lamarck, 1799 and O. carolinensis Verrill, 1884 from Cape Hatteras, United States, and O. vincenti Pickford, 1955 from São Vicente Island, Cabo Verde, although the latter was synonimised with A. burryi by Voss & Toll (1998). The type locality of A. burryi lies in the western Atlantic, along the coasts of Florida, with its distribution extending through the Florida Keys, the Gulf of Mexico, the Caribbean Sea, and down to Brazil (Fig. 1). Following its synonymisation with O. vincenti from Cabo Verde, records along the West African coast, from Senegal to Angola (Adam 1962), the species is also considered to be present in the eastern Atlantic (Fig. 1) (Mangold 1998, Voss & Toll 1998). However, the amphi-Atlantic distribution of A. burryi has been questioned by some authors and they doubt that the western and eastern forms really belong to the same species (Jereb et al. 2014). Most of the recent records of A. burryi along the eastern Atlantic coast are limited to Cabo Verde and the Canary Islands, where the species was first reported in 2013 from the island of Tenerife (Guerra et al. 2013). These records were based solely on photographs or morphometric analyses. Following the report from Tenerife, the species has been found on other Canary Islands (Lanzarote, Fuerteventura and La Palma) and is now frequently reported by scuba divers on citizen science platforms dedicated to marine species monitoring (e.g., RedPromar and iNaturalist).
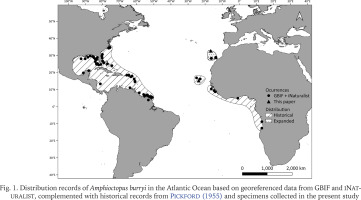
Fig. 1
Distribution records of Amphioctopus burryi in the Atlantic Ocean based on georeferenced data from GBIF and iNaturalist, complemented with historical records from Pickford (1955) and specimens collected in the present study
In this study, we report the first documented occurrence of A. burryi at Madeira Island and provide the molecular identification of several individuals from Cabo Verde, the Canary Islands, and Madeira using DNA barcoding techniques. Using these data, we confirm the amphi-Atlantic distribution of the species and discuss its possible recent northward expansion in the eastern Atlantic.
MATERIALS AND METHODS
SAMPLE COLLECTION
A total of four specimens of Amphioctopus cf. burryi were sampled, two of them were collected at Tarrafal, Santiago Island, Cabo Verde (15.2807, −23.7537), one from Playa Chica in Lanzarote Island, Canary Islands, Spain (28.9184, −13.6691), and one from Quinta do Lorde in Madeira Island, Portugal (32.7399, −16.7133) (Figs 2–4). All specimens were collected from sandy bottoms at depths ranging from 20 to 24 m. Tissue samples were obtained from the tips of the arms of each specimen and immediately preserved in 96% ethanol to ensure the integrity of DNA for subsequent molecular analyses. The complete specimens from Cabo Verde and from Madeira were preserved in alcohol and the specimen collected from Lanzarote was conserved frozen for posterior morphometric analysis. The specimens from Madeira and Lanzarote were deposited in the zoological collections of the Natural History Museum of Funchal (MMF) and the Natural History and Archaeology Museum (MUNA) of Santa Cruz de Tenerife, under collection references MMF 051316 and TFMCBM-MO/05209, respectively.
Figs 2–4
Amphioctopus burryi: 2 – from Playa Chica, Lanzarote Island, Spain, 14/08/2024; 3 – from Tarrafal, Santiago Island, Cabo Verde, 30/04/2024; 4 – from Quinta do Lorde, Madeira Island, Portugal, 12/08/2024. Photos: C. Camacho (2) and P. Wirtz (3, 4)

To visualise both historical and recent distributional records of A. burryi, a map was created using georeferenced presence data obtained from the Global Biodiversity Information Facility (Gbif.org 2025) and iNaturalist (2025). These records included those from Cabo Verde mentioned by Pickford (1955), as well as recent observations from Cabo Verde and the Canary Islands, including our sampled specimens. The map was generated using QGIS 3.34 ‘Prizren’ software (QGIS Development Team 2025).
MOLECULAR ANALYSIS
The DNA extraction was conducted using a portion of arm tissue with E.Z.N.A.® (V-Spin) kit (Omega Biotek, Georgia). The 5' region of the mitochondrial DNA (mtDNA) cytochrome c oxidase I (Cox-1) gene (658 bp) was amplified using universal primer pairs HCO2980/LCO1490 (Folmer et al. 1994). PCR conditions included an initial denaturation step at 94 °C for 3 minutes, followed by 40 cycles of denaturation at 94 °C for 30 seconds, annealing at 45 °C for 30 seconds, and extension at 72 °C for 1 minute, with a final extension at 72 °C for 10 minutes. PCR products were sent for sequencing to Macrogen service (Macrogen, Madrid). The resulting chromatograms were edited and aligned using MEGA11 software (Tamura et al. 2021). Species identification was verified with the ID engine in BOLD system v4 (Ratnasingham et al. 2024) and GenBank BLAST tool with (Benson et al. 2013) the public records of Amphioctopus genus. Finally, a maximum likelihood (ML) tree was made with all available A. burryi sequences of Cox-1 using the IQ-Tree web-page application (Nguyen et al. 2015) using Octopus vulgaris as an outgroup.
RESULTS
MORPHOLOGICAL ANALYSIS
The colour of living individuals varied from red-brown to light sandy or whitish on the dorsal side, with very distinctive dark brown to purplish brown longitudinal stripes along the dorsolateral margins of all arms (Figs 2–4). Grainy skin texture, covering the whole body including head, mantle and arms was also evident. Of the four specimens sampled, the individual from Madeira was a female, while the specimens from Lanzarote and the two from Cape Verde were males.
Arms unequal in length, with pair I shorter and slenderer than the rest, with pairs III and IV being longer and equal. Left arm I much shorter than the right (brachial formula 4 = 3 > 2 > 1). Well-developed brachial webs between arm pairs III and IV, occupying up to 30% of the arm length and very short dorsal webs. Arms with two rows of suckers without enlarged suckers. Right third arm hectocotylised in males, wider than the other arms at the base. Ligula with approx. 15 transversal folds. Calamus is short, with a narrow, open groove in its middle which connects to the spermatophoric canal on the arms side. The female specimen displayed the right third arm with a long, slender tip.
Eyes whitish or slightly reddish with a dark eye bar across the eye at the pupil (Figs 5–6, 8). Supraocular papillae are conspicuous, with a single large conical papilla located over the posterior dorsal region of each eye (Fig. 9), although these are not always visible in live individuals. Additionally, four longitudinal flaps arranged in a diamond pattern are present on the mid-dorsal mantle (Fig. 9), though they are also not consistently observed in live specimens.
Figs 5–10
Some colour and behavioural patterns exhibited by Amphioctopus burryi specimens from the Eastern Atlantic: 5 – specimen with a purplish-brown colour pattern on its arm stripes and eye bars, carrying sunglasses as shelter; 6 – specimen inside a 1L PET bottle, exhibiting a whitish body colour, a black eye bar, and black stripes on the arms; 7 – light sandy-coloured specimen with an apparent grainy skin texture; 8 – specimen with a whitish body colour and a distinct dark eye bar across the eye, using a soda can as a shelter; 9 – specimen showing extended supraocular papillae and dorsal medial flaps; 10 – specimen displaying stilt-walking behaviour while carrying a Pinna rudis shell remnant as shelter. Photos: M. M. Solá, La Tejita, Tenerife (5) and D. Rabeling, Playa Grande, Lanzarote (6–10)

BEHAVIOURAL NOTES
Both sampled and observed specimens typically exhibited a calm demeanour towards divers, either resting on the sand or taking shelter in objects. These shelters included natural items, such as mollusc shells (e.g., Patella spp., Pinna spp.), as well as marine litter (e.g., glass or plastic bottles, soda cans) (Figs 5–6, 8, 10). In some cases, individuals were observed engaging in a locomotion behaviour known as “stilt walking”, where the animal carries its shelter while moving. Observed examples included carrying marine debris, such as plastic sunglasses and fragments of Pinna spp. shells (Figs 5, 10). It was also common to observe specimens either partially or completely buried in the sand when divers approached too closely. Additionally, some individuals displayed bipedal locomotion to move away from divers. All these behaviours observed are shared with its congeneric species, the coconut octopus (A. marginatus) (Finn et al. 2009).
MOLECULAR CHARACTERISATION
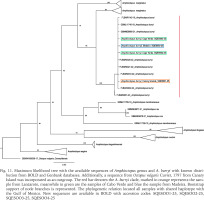
A 658-bp fragment was successfully amplified for each of the specimens related to Amphioctopus cf. burryi (BOLD accession code: SQES001-25, SQES002-25, SQES003-25, SQES004-25). All four collected specimens showed similarities between 99.69–100% (p-distance) to specimens of Amphioctopus burryi from the Gulf of Mexico, United States (accession code: FLBAR2320-22), confirming the amphi-Atlantic distribution of the species (Fig. 11).
Fig. 11
Maximum likelihood tree with the available sequences of Amphioctopus genus and A. burryi with known distribution from BOLD and Genbank databases. Additionally, a sequence from Octopus vulgaris Cuvier, 1797 from Canary Island was incorporated as an outgroup. The red bar denotes the A. burryi clade, marked in orange represents the sample from Lanzarote, meanwhile in green are the samples of Cabo Verde and blue the sample from Madeira. Bootstrap support of node branches is represented. The phylogenetic relation located all samples with shared haplotype with the Gulf of Mexico. New sequences are available in Bold with accession codes: SQESOO1-25, SQESOO2-25, SQESOO3-25, SQESOO4-25
DISCUSSION
The first putative specimen of Amphioctopus burryi recorded in the eastern Atlantic dates back to 1876. This specimen was collected during the HMS Challenger expedition near São Vicente Island, Cabo Verde, and was initially identified by Hoyle (1886) as Octopus granulatus. It was later designated as one of the paratypes of Octopus vincenti, a species described by Pickford (1955). Another specimen, collected in 1948 near Boa Vista Island, Cabo Verde, during the Piccard-Cosyns expedition, was initially classified as Octopus sp. by Adam (1952). This specimen was subsequently identified as the holotype of O. vincenti described by Pickford (1955). In her work, Pickford had already noted the similarity between O. vincenti and A. burryi, a species described from Florida a few years earlier by Voss (1950). This resemblance ultimately led to the synonymisation of O. vincenti with A. burryi (Voss & Toll 1998).
In this study, we have confirmed the amphi-Atlantic distribution of Amphioctopus burryi using molecular techniques. Voight (1998) noted the existence of at least six coastal octopuses’ species with an amphi-Atlantic distribution: Octopus vulgaris, Macrotritopus defilippi, Callistoctopus macropus, Pteroctopus tetracirhus, Scaergus unicirrhus, and Amphioctopus burryi. However, González-Gómez et al. (2024) suggest that many species from the American Atlantic coast have been misidentified due to a Eurocentric perspective, which often assumes the widespread amphi-Atlantic distribution of Eastern Atlantic species.
The use of molecular techniques, such as DNA barcoding, has helped clarify the taxonomy of amphi-Atlantic coastal octopuses by revealing cryptic speciation, showing that some species previously considered widespread actually represent distinct species on both sides of the Atlantic, such as the O. vulgaris species complex and C. macropus (Leite et al. 2008, Ritschard et al. 2019, Avendaño et al. 2020, Jesus et al. 2021). In contrast, A. burryi does not appear to follow this pattern of speciation; this could suggest either a recent colonisation or ongoing gene flow between the two sides of the Atlantic.
The archipelagos of Cabo Verde, the Canary Islands, Madeira, and the Azores – although belonging to three different marine ecoregions (Freitas et al. 2019) – exhibit a strong amphi-Atlantic component in their marine fauna. This pattern reflects an increasing gradient from the temperate archipelago of Azores to the more tropical Cabo Verde Islands, probably due to Equatorial Currents, which facilitate the dispersal of species between American and African coast (Wirtz & Martins 1993, Wirtz 2001, 2003). Transoceanic dispersal in other groups such as tropical reef fishes appears to be frequent and occurs along three main routes: from the Caribbean to the Northeast Atlantic, from northern Brazil to the Gulf of Guinea, and from Africa to southern Brazil (Muss et al. 2001, Joyeux et al. 2008). The ability of coastal tropical fishes to cross large biogeographic barriers is not solely dependent on larval dispersal capacities but also on the rafting capacity of adults (Floeter et al. 2008, Luiz et al. 2012). In the case of coastal merobenthic octopuses with small pelagic larvae, such as O. insularis, transatlantic dispersal has also been linked to Equatorial Currents, which connect both continents through westward and eastward flows (Amor et al. 2014, Lima et al. 2023). The role of rafting in juveniles or adult octopus dispersal cannot be ruled out, as some species have been observed rafting on natural objects such as kelps or pyrosomids far from coastal or continental shelves (e.g., Hobday 2000, Thiel & Gutow 2005, Villanueva et al. 2019).
By whatever means, A. burryi has demonstrated remarkable dispersal capacity, successfully colonising both sides of the Atlantic. In the western Atlantic, it is found from the coasts of North Carolina and Florida, extending through the Caribbean Sea to northern Brazil (Voss & Toll 1998). In the eastern Atlantic, it has been recorded from Senegal to Angola, including the Cabo Verde Islands, the Canary Islands and now Madeira Island (Fig. 1) (Voss 1951, Adam 1962, Voss & Toll 1998, Guerra et al. 2013, this paper).
The poleward expansion of marine species typically found in tropical and subtropical waters, which subsequently establish themselves in non-native temperate regions, is a phenomenon known as “tropicalisation” (Boero et al. 2008, Zarzyczny et al. 2024). This process has been particularly well documented across multiple marine taxa in the Canary and Madeira Islands (e.g., Brito et al. 2017, Falcón et al. 2018, Schäfer et al. 2019, Schäfer 2023, da Silva et al. 2023, Escánez & Camacho-Puerta 2024, González et al. 2025). The poleward expansion of cephalopods, likely driven by ocean warming, has been documented in various squid, sepiolid, and octopus species across different ocean basins, including in the eastern Atlantic (Guerra et al. 2002, Kim et al. 2012, Domínguez-Contreras et al. 2013, Golikov et al. 2013, 2014, 2024, Ruíz-Cooley et al. 2013, Ramos et al. 2018). These shifts suggest that warming oceans are facilitating the establishment of cephalopods beyond their historical ranges.
The successful establishment of octopus species beyond their native distribution ranges has been largely linked to their thermal tolerance (Ramos et al. 2018). This allows them to thrive within their thermal optimum and in the “pejus zone”, where performance is reduced but survival is still possible (e.g., Ángeles-González et al. 2020a). The thermal preferences and pejus zone of A. burryi remain unknown, but other physiological and environmental factors such as competition, predation, mobility and environmental barriers can also affect species distribution and establishment (Ángeles-González et al. 2020b, Ramos et al. 2023). Since its first record in 2013, A. burryi has become well-established in the Canary Islands, where it is frequently observed across both western and eastern islands (RedPromar 2024), suggesting that current environmental and biotic conditions fall within its tolerance range. Similar mean SST values in Madeira, and Canary Islands (17 °C in winter to 27 °C in summer) may support its spread, especially given the region's ongoing ocean warming, estimated at 0.2 °C per decade (Martins et al. 2007, Vélez-Velchí et al. 2015, Alfonso et al. 2021), which could facilitate its establishment in its northern limit and potentially its further expansion.
However, it is also possible that A. burryi has always been present in these regions as rare species at very low abundances, which reduces the likelihood of capture or observation using standard survey techniques such as fishing nets, traps, or, in our case, underwater visual censuses (Gu & Swihart 2004). This low detectability is further exacerbated in species with nocturnal and cryptic behaviours, such as A. burryi, and by the presence of abundant, morphologically similar native species such as O. vulgaris in the same habitats. Moreover, to confidently determine whether a species represents a new arrival, it is essential to have a comprehensive historical knowledge of the teuthofauna of the area. This, in turn, largely depends on the existence of extensive sampling effort and taxonomic work carried out by specialists on the group within that region (Nekhaev 2016). The absence of such knowledge often leads to species being overlooked and to erroneous inferences about their presence or distribution.
All these factors make it particularly challenging to categorise the occurrence of a new thermophilic species in subtropical waters as native or not, as these areas may represent the suboptimal, boundary zone of its distribution. Environmental changes, such as ongoing ocean warming, could be making the species less rare and increasingly abundant. This process would represent a case of “meridionalisation” – phenomenon described in the Mediterranean and which involves the local increase in abundance of a thermophilic species within its native range – rather than a true “tropicalisation”, which refers to the arrival of non-indigenous tropical species into temperate ecosystems (Bianchi & Mori 1993, Boero et al. 2008).
The rapid increase in observations of this species reported on citizen science platforms (e.g. iNaturalist, RedPromar) since its first record in 2013, and particularly from several Canary Islands after 2015, suggests that the species was already established there. Once its presence became known, divers quickly learned to recognise and differentiate it. Therefore, a similar pattern is expected to occur in Madeira, where the number of records from diving spots will likely increase in the coming years as awareness among divers grows.
This record brings the number of cephalopod species known from Madeiran waters to 78, including 16 octopods (Clarke & Lu 1995). Although it remains unclear whether A. burryi represents a recent arrival or a previously undetected resident, continued monitoring and integrative taxonomic work will be essential to detect early arrivals and to assess the population dynamics of rare species, as well as the ecological implications of such distributional or abundance shifts linked to ongoing ocean warming.

