INTRODUCTION
Rocky intertidal zones are some of the most physically demanding environments for marine organisms. To survive on rocky shores requires specialised adaptations to the cyclical exposure to air and immersion in water. Covering up to three-quarters of global coastlines (Johnson 2024), these habitats are shaped by vertical zonation, where the combined effects of various factors determine species distributions. Biotic factors such as the presence of epibionts (Pereira et al. 2022), predation pressure (Bazterrica et al. 2007, Silva et al. 2008), and competition for space and resources such as food (Boaventura et al. 2003, Holmes et al. 2005), shape community composition and spatiotemporal dynamics (Gerwing et al. 2016), particularly within the mid and low intertidal zones. These factors operate in conjunction with abiotic gradients, like variations in sunlight exposure, temperature, salinity, and desiccation risk (Connell 1961). Such abiotic stressors are particularly pronounced in tropical regions like the Philippines, where extreme summer temperatures and intense monsoonal rains challenge intertidal communities. Limpets, which are adapted primarily to seawater, experience significant physiological stress during prolonged exposure to freshwater from monsoon rains. This stress manifests in several ways, including alterations in heart rate, disruptions in osmoregulation, changes in gene and protein expression, and detachment from substrates (Morritt et al. 2007, Firth & Williams 2009, Dong & Williams 2011, Dong et al. 2014). Although data from the Philippines are limited, studies on similar tropical shores, like in Hong Kong, which experiences a strongly seasonal, monsoon-driven climate (Williams et al. 2019), have associated hot and wet summers with significant mortality events (e.g., Cellana grata (A. Gould, 1859)) resulting from desiccation, osmotic stress, and prolonged emersion (Williams & Morritt 1995). Therefore, comparable ecological impacts are likely to occur in the Philippines.
The pulmonate limpet Siphonaria javanica (Lamarck, 1819) (Family Siphonariidae) is a common gastropod known to influence ecosystems by regulating algal growth and shaping barnacle settlement (Santelices & Correa 1985). It typically inhabits the upper midshore area (Lee et al. 2009) or near the high tide line, like those found along seawalls on the coast of Singapore (Biswas & Bandyopadhyay 2014), but can extend into the supralittoral zone of wave-exposed rocky shores, such as in Antique, Philippines (Putong & Villarta-Lane 2024). Bazterrica et al. (2007) found that about 90% of pulmonate limpets inhabit cracks and crevices during low tide, which aids survival by providing refuge from harsh environmental conditions (Gray & Hodgson 1998, Jones & Boulding 1999). In Thailand, Siphonaria guamensis Quoy & Gaimard, 1833 was more abundant on vertical surfaces at higher, hotter shore levels, suggesting species-specific morphological or physiological adaptations (Sangphueak et al. 2024) and activity patterns that help cope with desiccation (Zapanta et al. 2020). While morphological and behavioural adaptations like ridged-shells and clamping are well-documented (Harley et al. 2009), physiological responses to stressors, especially hypo-osmotic conditions, are less studied. For instance, Siphonaria oculus Krauss, 1848 heart rate correlates strongly with ambient temperature, showing day-night variation but no seasonal metabolic compensation (Marshall & McQuaid 1994). It has also shown desiccation tolerance, which likely stems from metabolic rate depression – an energy-saving strategy enabling survival during extended emersion (Marshall & McQuaid 1992, 1994). This underscores the importance of investigating how changing environments affect organisms’ physiological responses (Helmuth 2009).
Physiological metrics, such as heart rate (HR), provide important insights into the metabolic responses of marine invertebrates to environmental challenges (DeFur & Mangum 1979, Braby & Somero 2006). HR monitoring is a non-invasive method increasingly applied in marine ecological studies and has proven effective in assessing the impacts of stressors such as temperature and salinity (Somero 2002, Williams et al. 2019). This has been shown in bivalve species such as the blue mussel Mytilus trossulus A. Gould, 1850 and M. edulis Linnaeus, 1758 (Bakhmet et al. 2022) and green mussel Perna viridis (Linnaeus, 1758) (Nicholson 2002), as well as in other non-marine gastropods like the garden snails Cornu aspersum (O. F. Müller, 1774) (Bruning et al. 2013) and Batillaria attramentaria (G. B. Sowerby II, 1855) (Han et al. 2023).
These methods have revealed that stress responses often involve metabolic trade-offs, with some species suppressing aerobic metabolism to conserve energy during prolonged exposure to adverse conditions (Marshall & Mcquaid 1992), such as the heat stress experienced by Cellana spp. H. Adams, 1869 (Virgin & Schiel 2023). However, most of these investigations have focused on temperate species, leaving a gap in our understanding of how tropical intertidal gastropods, like S. javanica, physiologically adapt to their environment. The present study aimed to address this gap by establishing baseline HR data for S. javanica in the Philippines. The potential influence of temperature and rainfall on metabolic responses was also explored.
MATERIAL AND METHODS
The study was conducted in October 2024 during a spring tide on a semi-exposed rocky shore in southern Antique, Philippines (10.50500°N, 121.92058°E). Five S. javanica individuals of different sizes (shell lengths: 2.18–15.6 mm) were monitored. Heart rate data were analysed by calculating basal HR, defined as the average heart rate at rest for each individual, and mean HR, calculated as the average of all recorded heart rate measurements for each limpet across the entire sampling period.
Low tidal amplitude (~1 m above Chart Datum) and reduced wave action provided optimal conditions for in situ recordings. Limpets inhabited the mid- to upper shore (~1.5 m above C.D.), favouring microhabitats, such as depressions and small crevices. During sampling, all limpets were inactive and intermittently exposed to rainfall, with no seawater immersion. HR was monitored following the non-invasive technique described in Burnett et al. (2013) using a Heart Frequency Logger (ElectricBlue PULSE V2 10-channel). This is a heart rate monitor that uses infrared sensors (Vishay CNY70, 7 × 7 mm; QRE1113, 2 × 2 mm) to detect and log cardiac activity (Supplementary Fig. 1). Infrared sensors were securely attached to the limpets’ shells above the heart using Blu Tack® (Supplementary Figs 2–3). HR measurements were recorded from 10:00 to 16:00. Mean HR (beats per minute) was calculated from five-minute readings every 30 minutes. Rock surface temperature, ambient air temperature, and adjacent seawater temperature near each limpet were also monitored using thermocouple wires attached to a digital thermometer.
All data analyses were performed using R version 4.5.0. Linear regression was used to test for the size effect on HR and assess proportional scaling. A generalised additive mixed model was applied using the mgcv package to test immediate HR responses to rock temperature fluctuations. A separate linear mixed model was fitted to HR measured at four sampling intervals after each rock temperature reading to test whether rock temperature exerted a delayed effect on HR. Model fit and significance were assessed using t-values, and variance explained was reported via marginal and conditional R² values using the performance package. To explore temporal dynamics, HR and temperatures were standardised (z-scores) and plotted. This approach removed the confounding influence of absolute HR differences due to body size and allowed clearer inference of within-individual physiological responses to temperature differences.
RESULTS AND DISCUSSION
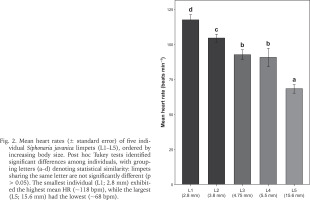
On the day of the field assessment, weather conditions varied, with partly cloudy skies and light/heavy rainfall between 11:00 and 12:00 followed by light rain showers lasting about 20 minutes after 13:00. The linear regression showed a significant inverse relationship between limpet size and mean HR (slope = –3.13 bpm mm–¹, R² = 0.471, p < 0.001) (Fig. 1). ANOVA and Tukey’s post-hoc tests corroborated distinct HR groups among the sampled individuals, from 117.6 ± 2.2 bpm in the smallest limpet (L1) to 68.4 ± 2.0 bpm in the largest (L5) (Fig. 2). This trend aligned with the size-dependent HR patterns seen in other limpets (Chelazzi et al. 1999, Santini et al. 1999, 2000) and intertidal species, such as the shore crab Carcinus maenas (Linnaeus, 1758) (Ahsanullah & Newell 1971) and sea slugs (Gibson 2019), as smaller individuals typically exhibit higher metabolic rates due to their larger surface area-to-volume ratios (Vermeij 1973, Glazier 2005).
Fig. 1
Relationship between body size and heart rate in Siphonaria javanica. Each point represents an individual five-minute mean heart rate measurement plotted against shell length (2.8–15.6 mm). HR decline linearly with shell length (2.8–15.6 mm); HR = 114.9 − 3.13 × length (95% CI shown). The slope (−3.1 bpm mm−1) is highly significant (t = −14.7, p < 2 × 10−16) and explains 47% of the variance (R2 = 0.47).
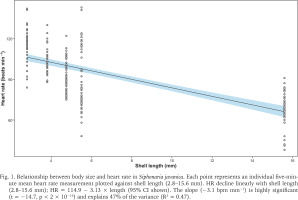
Fig. 2
Mean heart rates (± standard error) of five individual Siphonaria javanica limpets (L1–L5), ordered by increasing body size. Post hoc Tukey tests identified significant differences among individuals, with grouping letters (a–d) denoting statistical similarity: limpets sharing the same letter are not significantly different (p > 0.05). The smallest individual (L1; 2.8 mm) exhibited the highest mean HR (~118 bpm), while the largest (L5; 15.6 mm) had the lowest (~68 bpm).
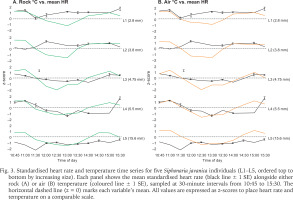
Heart rate fluctuations throughout the day followed a distinct pattern, with a notable decrease around midday (11:30–12:00) across all size classes during the rainfall event (Fig. 3). This drop coincided with decreases in air and rock temperatures, suggesting a physiological response to thermal changes or a behavioural adaptation to conserve energy.
Fig. 3
Standardised heart rate and temperature time series for five Siphonaria javanica individuals (L1–L5, ordered top to bottom by increasing size). Each panel shows the mean standardised heart rate (black line ± 1 SE) alongside either rock (A) or air (B) temperature (coloured line ± 1 SE), sampled at 30-minute intervals from 10:45 to 15:30. The horizontal dashed line (z = 0) marks each variable’s mean. All values are expressed as z-scores to place heart rate and temperature on a comparable scale.
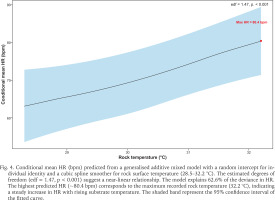
Rock temperature plays an important role in shaping S. javanica thermal stress response. Generalised additive mixed model analysis indicated a near-linear increase in HR with rising rock temperature (edf = 1.47, p < 0.001), with maximum HR (~80.4 bpm) at the highest temperature observed (32.2 °C). The model accounted for 62.6% of HR variability (Fig. 4). During rainfall events, limpets tightly clamp onto rock surfaces to minimise rainwater contact—a behavioural adaptation that enhances their survival prospects until the rain stops or till the subsequent tidal immersion. Additionally, limpets mitigate stress by seeking cooler spots like crevices or vertical surfaces (Garrity 1984, Levings & Garrity 1984, Gray & Hodgson 2004, Virgin & Schiel 2023). Despite providing refuge from adverse environmental conditions, these microhabitats can still become stressful, particularly during periods of rainfall. Marine organisms residing in crevices or even in tidepools can be completely inundated by freshwater (Firth & Williams 2009), leading to periods of extreme environmental stress. The review by Dong (2023) posited that small-scale thermal variations can affect physiological performance. For instance, microclimates in sheltered microhabitats can exceed air temperatures, which can exacerbate heat stress and influence heart rates. Lastly, limpets exhibit short-term, high-risk behaviours like “mushrooming” in which they lift their shells off the rock as a last resort to relieve thermal stress (Williams & Morritt 1995, Williams et al. 2005).
Fig. 4
Conditional mean HR (bpm) predicted from a generalised additive mixed model with a random intercept for individual identity and a cubic spline smoother for rock surface temperature (28.5–32.2 °C). The estimated degrees of freedom (edf = 1.47, p < 0.001) suggest a near-linear relationship. The model explains 62.6% of the deviance in HR. The highest predicted HR (~80.4 bpm) corresponds to the maximum recorded rock temperature (32.2 °C), indicating a steady increase in HR with rising substrate temperature. The shaded band represent the 95% confidence interval of the fitted curve.
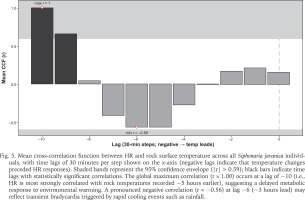
Post-rainfall, the HRs of all limpets gradually increased as temperatures rose, possibly reflecting a physiological response to rising thermal stress, a phase of recovery, or a combination of both. Cross-correlation analyses revealed the strongest HR response to rock surface temperature at a lag of approximately 5 hours (r ≈ 1.00), and a negative correlation (r ≈ −0.56) at a 3-hour lead, indicative of delayed metabolic adjustment and transient bradycardia (Fig. 5). These results suggest that S. javanica adapts to dynamic intertidal conditions but exhibits a delayed response to rapid salinity and temperature shifts. This was consistent with the observed bradycardia in Siphonaria capensis Quoy & Gaimard, 1833 under hyposaline conditions, with recovery possible under normal conditions (Marshall & McQuaid 1993). Similarly, M. edulis displayed temporary bradycardia following exposure to hyposalinity (15 ppm) but recovered upon returning to normal salinity levels (Bakhmet et al. 2005).
Fig. 5
Mean cross-correlation function between HR and rock surface temperature across all Siphonaria javanica individuals, with time lags of 30 minutes per step shown on the x-axis (negative lags indicate that temperature changes preceded HR responses). Shaded bands represent the 95% confidence envelope (|r| > 0.59); black bars indicate time lags with statistically significant correlations. The global maximum correlation (r ≈ 1.00) occurs at a lag of −10 (i.e., HR is most strongly correlated with rock temperatures recorded ~5 hours earlier), suggesting a delayed metabolic response to environmental warming. A pronounced negative correlation (r ≈ −0.56) at lag −6 (~3 hours lead) may reflect transient bradycardia triggered by rapid cooling events such as rainfall.
Rapid environmental shifts, such as heavy rain followed by high temperatures, have been linked to increased mortality in limpets (Morritt et al. 2007, Firth & Williams 2009, Harley et al. 2009, Williams et al. 2011). Smaller limpets are especially at risk during these events, owing to their limited ability to regulate salinity and their increased likelihood of detachment from rocky substrates (Morritt et al. 2007). The effects of rainfall on limpets are multifaceted, involving heart rate variability, shifts in metabolic responses, difficulties in osmoregulation, and interactions with numerous other environmental stressors (Firth & Williams 2009). Understanding these impacts is essential to predict how limpets and other intertidal species will adapt to large-scale environmental changes, such as those driven by climate change, ultimately shaping marine ecosystem resilience.
This study is the first in the Philippines to successfully measure the heart rates of S. javanica in natural shore conditions. As a result, the basal HR was determined, and we were able to show an inverse relationship between heart rate and shell size. The observed delayed HR response to rock temperature also shows the importance of considering physiological lags in ecological modelling. We recommend that future studies use increased sample sizes and more replicates for better representation. Comparison of HRs under various weather conditions would also benefit our understanding of thermal and osmotic responses in tropical intertidal limpets.




