INTRODUCTION
Giant African Land snails are a group of very large hermaphrodite land snails in the family Achatinidae (Ngowsiri et al. 1989, Egonmwan 2004). As with most land snails, they are active mostly at night, or in wet or overcast weather, but remain inactive when it is dry (Amusan & Omidiji 1999, Omole 2001, Akinnusi 2014). They are a commercially important item of diet in Nigeria and elsewhere, and A. marginata is now farmed. Snail meat has become so popular over the years that the supply can no longer keep up with the demand (Murphy 2001, Ebenso 2003, Paoletti 2005).
It is known that the reproductive systems of some land snails vary in size and chemical composition with season. Kotsakiozi et al. (2016), found that three Codringtonia species exhibited low lipid levels in their genitalia during spring, maintaining these concentrations at similarly low levels throughout the summer aestivation period. Sodipe et al. (2012) found variation in penis width, at a maximum in the rainy season in which the snails mated.
Within the reproductive system, the albumen gland plays a key role in providing nutrients to the eggs (Bayne 1973, Boyle & Yoshino 2000, Kiehn et al. 2004, Mukai et al. 2004, Ademolu et al. 2013), Its activity may be stimulated by a neurohormone produced by the brain in Helix pomatia (Goudsmit 1975). As vertebrate-like sex steroids such as progesterone, oestrogen and testosterone have been reported in Lissachatina fulica (Bose et al. 1997), Octopus vulgaris (Maha et al. 2009) and Biomphalaria alexandrina (Maha et al. 2009), assays of their levels in the albumen gland, a relatively large organ, over the seasons might be reliable indicators of A. marginata reproductive condition. This study monitored these changes, with the goal of aiding better management of A. marginata cultures in Abeokuta Ogun State, Nigeria.
MATERIAL AND METHODS
The snails used in this study were collected from the wild in Abeokuta, Ogun State, within the Rain Forest Zone of Southwest Nigeria. 240 adult A. marginata, weighing between 124 and 192 g, were placed in snail housing made up of three earthen cages (80 in each), each 8 ft by 6 ft, filled with loamy soil, set up at the Snail Research Unit of the Department of Pure and Applied Zoology, College of Biosciences, Federal University of Agriculture, Abeokuta, Ogun State.
The snails were left to acclimatise for 3 weeks after collection (Ola et al. 2016). They were fed with ripe and unripe pawpaw fruits, watermelon leftovers and fresh pawpaw leaves. The snails were fed ad libitum, daily and remnants of the previous day’s feeding were cleared before supplying any new feed to avoid contamination and attracting insects. After serving their feed, the snails were covered with materials such as, leaves of plantain and banana as mulch to help preserve moisture.
Sample Preparation and Laboratory Analysis
A total of 20 snails were selected randomly every month for twelve months (Giokas et al. 2005), they were dissected, their albumen glands were separated, and samples of the albumen gland were analysed using ELISA kit (Monobind, USA) for the determination of follicle stimulating hormone, oestrogen, progesterone, luteinizing hormone and testosterone concentrations. The units of measurement differ among hormones, following standard practice, and are indicated in Table 1. Because single snails do not yield enough material to conduct all five assays, an estimate of error was obtained by separating the 20 snails into three lots (seven, seven and six snails) for bulk analysis. The estimated error necessarily combines instrumental error and any differences between lots; it is generally very small (see below).
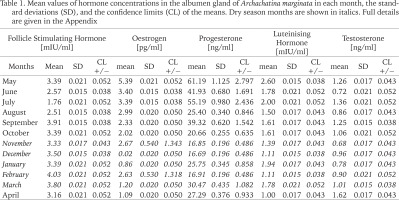
Table 1
Mean values of hormone concentrations in the albumen gland of Archachatina marginata in each month, the standard deviations (SD), and the confidence limits (CL) of the means. Dry season months are shown in italics. Full details are given in the Appendix
Statistical Analysis
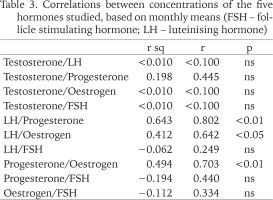
Examination of the data demonstrated that variation among the three subsamples in each month was usually very low (Appendix), and variation among months was often highly significant. While we have grouped months by season (wet: April to October; dry: November to March), it is important to note that there are significant differences between months in each, and some overlap in mean values. We have used Pearson two-tailed correlation analysis to study co-variation among hormones and between hormones and environmental factors, specifically, monthly data on rainfall and mean temperature, derived from the Weather Station, of the Department of Water Resources Management and Agrometeorology (WRMA), Federal University of Agriculture, Abeokuta, Ogun state Nigeria.
RESULTS
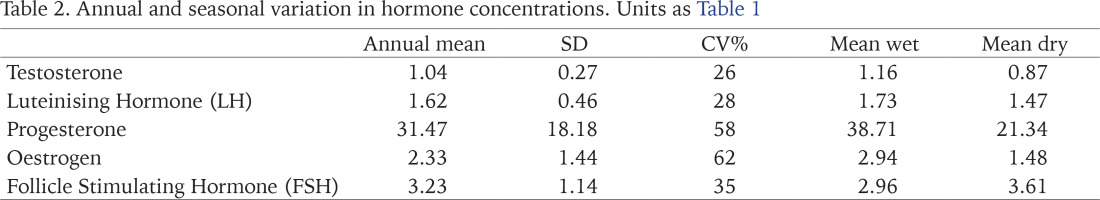
Changes in the concentration of the hormones tested in the albumen gland of A. marginata across the year are shown in Table 1. Details of individual measurements and error estimates are given in the Appendix. In general, confidence limits for the means in each month are very narrow, but we note two apparently anomalously large differences in individual values for oestrogen (in November and February; see Appendix). Further, there is a very low mean value for oestrogen in December, and the coefficient of variation for this hormone is the largest (Table 2). While there are differences in the mean values for the wet and dry seasons (Table 2), there is overlap in the values for individual months within each period, and there are also significant differences among months within each. While levels of LH, oestrogen and progesterone were strongly and positively correlated (Table 3), concentrations of testosterone and FSH varied independently of these and of each other. Peaks at the beginning of the wet season (April or May) were found in all but FSH. In relation to weather records for each month (data not shown), there were no significant relationships; all but FSH showed weak positive associations with rainfall, and weak negative associations with mean temperature.
DISCUSSION
The results of this study revealed that the vertebrate-like hormones (follicle stimulating hormone, oestrogen, progesterone, luteinising hormone, testosterone) were present and affected by seasonal changes in the snails studied. This is in agreement with work done by Alon et al. (2007), who also observed that these hormones show seasonal variations in the snails studied. It was also observed in this study that majority of the hormones (oestrogen, progesterone, luteinising hormone and testosterone) were at a peak in the first two months of the rainy season (May and April). This can be related to the start of reproductive activities during the rainy season. The increase in oestrogen, progesterone and luteinising hormone in the rainy season (especially in May) relates to their sexual activeness and the release of gametes from the male and female gonads during this period enhanced by favourable weather conditions (Wang & Croll 2004). According to earlier research, constant watering tends to increase the luteinising hormone concentration during pre-spawning; Omoyakhi et al. (2017) also report an association between oviposition and rainfall. Di Cosmo et al. (2001) showed that progesterone levels fluctuated according to the reproductive cycle in molluscs, being very low during the non-vitellogenic period (dry season) and increasing at the onset of vitellogenesis (usually rainy season). This hormone also shows the greatest difference between wet and dry seasons in our study. There are other reports of seasonal variation in reproductive hormones in molluscs (Reis-Henriques et al. 1990).
Two hormones, testosterone and FSH, however, did not conform to this pattern, nor did they vary in parallel. While Gooding & Leblanc (2005) reported that molluscs frequently exhibit low free testosterone levels during the non-reproductive phase and high testosterone levels in conjunction with the reproductive cycle, variation in this hormone between seasons was slight in our study, and had a low coefficient of variation overall. In contrast, FSH was unique in being at higher concentrations in the dry season, and in having a minimum in the middle of the wet season. It may be that the hormone relates to post-reproduction, gonadal tissue recovery, sustenance, or both, and it is also consistent with research by Okhale et al. (2018). In the same Archachatina marginata species Okhale et al. (2015) found increased FSH levels before aestivation (post-spawning), i.e. later in the wet season. Previous studies have also shown that weather conditions affect concentration of hormones (Salles 2010), even harsh weather conditions could lead to changes in the release of these reproductive hormones in animals (Wingfield et al. 1990).
While our results do indicate the expected relationships among hormones, and, broadly, between these and weather conditions and seasons, we note the sometimes great and significant differences between adjacent months in the same season. Aside from the possible effects of the experimental conditions, it may be that hormone levels respond to short term variation in weather conditions within months. There is scope for further work, possibly using snails collected directly from the wild, with data on the weather immediately preceding collection.