INTRODUCTION
The golden mussel, Limnoperna fortunei (Dunker, 1857) (Bivalvia; Mytilidae) known as “the golden menace” has been considered the third freshwater aquatic invasive species from Southeast Asia (native to rivers and streams in China) to invade South America ecosystems through the Río de la Plata Estuary, together with Corbicula fluminea (Müller, 1774) and Corbicula largillierti (Philippi, 1844) (Pastorino et al. 1993, Darrigran 2002). Ituarte (1981) suggested that both species of Corbicula were introduced between 1965 and 1975, while L. fortunei was detected in 1991, in each case following an increase in Argentinian imports from Hong Kong and Korea (Darrigran & Pastorino 1995). Most likely they have been transported through interoceanic vessels in ballast tanks containing untreated freshwater, the most common cause of introduction of aquatic invaders worldwide (Darrigran & Pastorino 2004, Darrigran et al. 2020, Lucía et al. 2023). The golden mussel has a rapid reproductive cycle and unique ability to cling to surfaces, obstructing pipes carrying water for the cooling systems within hydroelectric power plants. No native predators have been recorded, but the species is known to have ecological impacts such as growing on top of native molluscs, crustaceans, plants, etc. (Ludwig et al. 2020, Lucía et al. 2023), similar impacts to those caused by the zebra mussels Dreissena polymorpha (Pallas, 1771) infesting the Great Lakes in the United States (Ożgo et al. 2020), but much more widespread.
The economic cost caused by invasive species is estimated at 143 billion dollars a year in Brazil, and CEMIG, the largest energy trader in the country, has invested more than R$ 10 million in projects to moderate the invasion problem caused by the golden mussel (Silva et al. 2016).
Mansur et al. (2003) reported in 1998 a specimen of the golden mussel found at the Chico Inglês Island followed by several other reports from 1998 to 2000 spread around the Jacuí River Delta, state of Rio Grande do Sul. In addition, in 1998, Darrigran & Ezcurra-De-Drago (2000) recorded the occurrence of the golden mussel in the Paraguay River, in Corumbá, state of Mato Grosso do Sul (Avelar et al. 2004). Furthermore, the golden mussel was observed for the first time in the Itaipu Binacional hydroelectric power plant in 2001 (Canzi et al. 2014), located at the frontier between Brazil and Paraguay, thus beginning its way up the Paraná River Basin. Once in this river, the golden mussel continued its dispersion both actively and passively (mainly due to commercial navigation) being registered in the major upstream tributaries (Paranapanema, Tietê, and Grande) and in associated hydroelectric dams (Avelar et al. 2004, Oliveira et al. 2004, Pareschi et al. 2008). The confluence of the Grande and Paranaíba Rivers, at the upper basin of the Paraná River, occurs on the tri-border area between the states of Mato Grosso do Sul, São Paulo and Minas Gerais. The Paranaíba River runs further up north, along the south borders of the Cerrado biome. The Cerrado is a global biodiversity hotspot, one of the richest of all tropical savanna regions, with high levels of endemism and the second largest biome of Brazil, after the Amazonian rainforest.
Campos et al. (2012) studied a small stretch of the lower Paranaíba River where, among its tributaries of the right bank, including the São Simão reservoir, the golden mussel was only found in the Aporé River. They estimated a mean advance average of 14 km per year of golden mussel invasion, similar to the speed indicated by Boltovskoy et al. (2006) (an average rate of about 10–20 km per year). Sylvester et al. (2005) had indicated that the geographic reach of the golden mussel extended beyond 3,000 km from the first point of entry, in Argentina. In 2016, the presence of L. fortunei larvae was confirmed in water samples collected in the Emborcação reservoir (CBEIH 2015), located in the Paranaíba River, being the most northern record in the Paraná Basin to date. Furthermore, the presence of the golden mussel was observed in the low-middle São Francisco River, in the Sobradinho reservoir, state of Bahia in October 2015, a first record outside the areas of influence of the infested basins (CBEIH 2015).
The golden mussel was the first agglomerating organism to infest tropical freshwaters of South America (Sardiña et al. 2008, Darrigran et al. 2020). Since 1995, several studies have shown environmental and economic damages caused by incrustations in channelled water structures. With the expansion of the golden mussel invasion, several systems essential for the operation of hydroelectric power plants have been affected by the macrofouling effect, which causes clogging and blockage of filters, grids, heat exchangers, condensers and other industrial structures and equipment (Zanella & Marenda 2002, Darrigran & Mansur 2006, Darrigran et al. 2009, Darrigran & Damborenea 2009).
Besides being an international biodiversity hotspot, the Cerrado biome covers three of the largest watersheds in South America, contributing 43% of Brazil surface water outside the Amazon basin. The Cerrado is a key biome for Brazil economic development, worldwide food production, maintenance of water cycles, preservation of biodiversity and for global climate change mitigation and adaptation. Despite its enormous importance for species conservation and the provision of ecosystem services, the Cerrado has lost 88 Mha (46%) of its native vegetation cover, and as little as 19.8% remains undisturbed (Strassburg et al. 2017). As a much-overlooked threat, knowledge about the invasive bivalve species in the rivers of Central Brazilian Cerrado is scarce.
In aquatic environments, organisms such as bivalves or fish transported from one place to another (outside their distribution range) can cause severe economic (decrease in fishery production for native species) and ecological impacts, as discussed above. Similarly, if a native species is transferred or translocated from one body of water to another (even within its distribution range) (Mejía-Mojica et al. 2012) and it finds favourable conditions, it can produce a similar impact as an invasive alien species.
Accordingly, the aim of this study was to report an extension of the geographical distribution of the golden mussel and enlarge the list of the invasive species that have arrived in the Cerrado (Brazilian savanna) environment. We also aimed to provide a biometric analysis of the populations found and histological characterisation of the gonads of male and female individuals.
MATERIAL AND METHODS
STUDY AREA AND DATA COLLECTION
The golden mussels (n = 386) were collected manually in the Paranaíba River at the Central Brazilian Cerrado on June 5th, July 25th and August 22nd, 2017. The study area was the middle Paranaíba River, located in and around the hydroelectric power plant of Cachoeira Dourada, namely at 18°30'01.9"S, 49°29'29.0"W, 18°30'35.4"S, 49°30'02.3"W, 18°29'59.5"S, 49°28'41.7"W and 18°37'47.0"S, 49°55'50.0"W. After collection, the mussels were transported alive (at 4 °C) to the laboratory (Laboratory of Environmental Biotechnology and Ecotoxicology – LaBAE) at Federal University of Goiás, Brazil. Analyses were undertaken 4 hours after collection and some mussels were kept in 4 L tanks with water at 26 ± 1 °C and a 12:12 h photoperiod (light/dark).
To assess the mussel physiological condition, 30 animals, at different shell length classes, were randomly collected for condition index (CI) determination, while another 15 mussels were analysed by histology. The identification of mussels was based on characteristics described by Pereira et al. (2012). Animal care and handling were carried out by institutional guidelines according to Brazilian laws by a Permanent License for Collections of Zoological Material number 34144-1 by the Biodiversity Authorization and Information System – SISBIO.
BIOMETRY AND CONDITION INDEX
In the laboratory, each mussel was measured with a digital calliper for the shell length (maximum distance along the anterior posterior axis; mm), shell width (maximum lateral axis; mm) and height (maximum dorso-ventral distance perpendicular to the hinge line). The total weight (g) and the whole soft tissue weight (g) of mussels were determined.
The CI was determined to indicate the physiological state of the organisms and is directly proportional to the amount of stored energy. It was calculated as the percentage (%) of the ratio between drained weight of the whole soft tissues (g) and total weight (g), according to Galvão et al. (2000).
HISTOLOGY
The mantle and the reproductive system (n = 15) were carefully dissected and immediately fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (PBS) at pH 7.2. Afterwards, portions of organs were washed in PBS buffer (0.1 M, pH 7.2), dehydrated in ethanol gradient, embedded in paraffin (Paraplast/McCormick), sectioned (5 µm thick) and stained with haematoxylin and eosin (H&E) (10 sections per animal). Histological analysis and digital image capture were performed with a light microscope associated with a digital camera (Leica DM500) and the Leica Application Suite software. The reproductive stages (developing, maturing, mature and spent) were identified according to Boltovskoy et al. (2015).
STATISTICAL ANALYSIS
All the statistical analysis was conducted using the software GraphPad® Prism 6 for Windows, version 6.05. The Bartlett test, with a significance level α of 0.05, was used to verify homogeneity of variances (H0) across the sampled material. Once the null hypothesis was rejected, Kruskal-Wallis H test was the non-parametric method used, together with Dunn’s multiple comparison test post hoc, to compare the data obtained.
RESULTS AND DISCUSSION
In total, 386 mussels were captured from four different locations of the Paranaíba River in Central Brazilian Cerrado (Fig. 1). The individuals analysed at site I (power plant) corresponded to 53.9% (n = 208) of the mussels; site II (water treatment station) 5.2% (n = 20); site III (fish farm upstream) 28.5% (n = 110); and site IV (fish farm downstream) 12.4% (n = 48).
Fig. 1
Golden mussel Limnoperna fortunei collected from June to August 2017 in four locations at the mid Paranaíba River. Observe shells with different length classes. Scale bar 10 mm

Shell length of the 386 specimens ranged from 3.8 to 35.5 mm (15.73 ± 5.5 mm); width from 1.9 to 14.1 mm (7.20 ± 2.2 mm); and height from 1.4 to 11.8 mm (5.8 ± 2.0 mm) (Fig. 2). According to Boltovskoy & Cataldo (1999), at one year of life individuals reach approximately 20 mm in length; by the end of the second year, they reach 30 mm long, and the maximum theoretical length is 35 mm by the third year. In the present study, 78.2% (n = 302) of the specimens captured presented shell length under 20 mm, 20.5% (n = 79) between 20 and 30 mm, and 1.3% (n = 5) were over 30 mm, indicating that the population has successfully been established for at least 3 years. Based on the measurements of the bivalves obtained, the present study indicates that the populations discovered are likely in its third year of establishment (invasion of 2014) and presents a high recruitment rate due to the presence of smaller shell length class individuals predominantly in the population.
Figs 2–3
Biometry of Limnoperna fortunei from mid Paranaíba River: 2 – relative frequency distribution in shell length classes; 3 – shell length (mean ± standard deviations) of L. fortunei from different areas. Abbreviations: aa – fish farm upstream, ff – fish farm downstream, pp – power plant, wt – water treatment
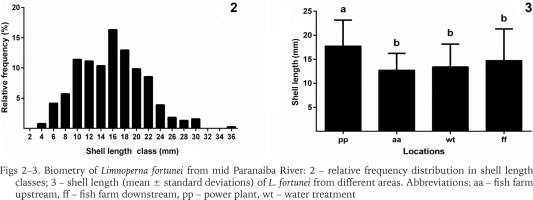
Furthermore, results indicate a difference in shell length between the areas (Fig. 3). The power plant showed the highest mean shell length, statistically differing from the other areas (H = 71.35; p < 0.001). This may probably be because from these places, only the unit generator presents a non-stop and continued water flow, which allows the mussels to remain encrusted for longer periods.
The Condition Index (CI) was obtained only for individuals found in the lower access hatch of the unit generation UG8 and ranged from 15 to 38.3%.
This high CI in the golden mussels collected from Paranaíba River indicates good physiological condition of the mussels collected. As pointed by Galvão et al. (2000), the CI may be related not only to nutritional and sanity aspects, but also to the reproductive cycle, when the gonads filled with gametes have higher rate of glycogen storage, resulting in high CI.
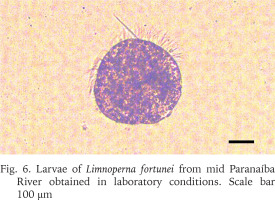
Moreover, different reproductive phases were observed, such as maturing, mature, and spent (Boltovskoy et al. 2015). The mantle from a male and a female individual were analysed and the histological sections for both (Figs 4–5) demonstrate gonads at a mature stage, with the oocytes filling up the follicles and the spermatocytes associated to the follicle lumen, indicating that the population has both males and females capable of reproduction. In addition, when some of the mussels collected were kept in laboratory for acclimatisation, two separate spawn events were observed, which resulted in the production of larvae (Fig. 6), reinforcing the population capability of reproduction.
Figs 4–5
Photomicrographs of the gonads from a female (4) and a male (5) of Limnoperna fortunei collected from June to August 2017 from mid Paranaíba River. Abbreviations: eg – epithelium germinal, lu – lumen, n – nucleus, nc – nucleolus, oc – oocyte, pt – pallial tissue, st – spermatids, tt – testicular tubules, v – vittelus, z – spermatozoa. Hematoxylin and eosin staining; 40× magnification and scale bar 100 μm
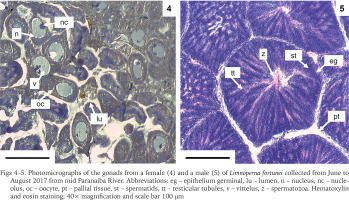
Fig. 6
Larvae of Limnoperna fortunei from mid Paranaíba River obtained in laboratory conditions. Scale bar 100 μm
Considering the range of the shell length plus the histology observations and larvae production, golden mussel population seems to be reproducing actively, which, in time, will most certainly lead to higher densities. Based on the measurements of the bivalves obtained, we could infer that the populations discovered in the mid Paranaíba River are likely in their third year and present a high recruitment rate due to the presence of various individuals of small shell length class. The occurrence of a stable population of golden mussel, which might be reproducing all year round same as in China (Yang et al. 2023) and raises high concern especially for the five major hydroelectric dams located in the Paranaíba River. According to the Operador Nacional do Sistema Elétrico – ONS (responsible for coordination and control of the generation and distribution of electric energy in Brazil), the Southeast and Middle East region, here including the Paranaíba and Paraná Basins, nowadays account for approximately 27% of the hydroelectric power produced and transmitted through the Brazilian Electric Sector.
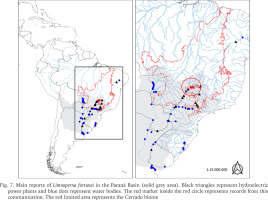
The main reports of L. fortunei in the Paraná Basin and the first record in the mid Paranaíba River are shown in Fig. 7 and Table 1, indicating that the golden mussel continues to colonise man-made structures associated with water systems, and strongly suggesting that the dispersion upstream in the river, even across dams, is aided and boosted by unsupervised expansion of fish farms. The increased presence of the golden mussel in Brazil has been linked to developments from anthropogenic activities (aquaculture, agriculture, etc.) delivering cyanobacterial blooms, nutrient recycling, and in some cases increased transparency of the water column (Boltovskoy & Correa 2015), according with aquaculture activities have increased in Brazil in the last decade and this raises the major concern that the invasion of the Amazon basin by the golden mussel may be catastrophic (Ludwig et al. 2020, Silva De Paula et al. 2021). Thus, it is urgent that the government starts considering it within the politics of authorisation and demands proper monitoring and reports from the fish farmers throughout the production chain. The absence of reports about the presence of the golden mussel in the other basins may be due to poor or even no monitoring. There are other challenges facing monitoring of the mussel invasion process. For example, early larval stages may be difficult to identify since the invasive L. fortunei larvae are similar to those of native mollusc species. In this context, most of the distinctive characters of L. fortunei can be distinguished only by trained specialists in the latest stages of the development using literature. In these cases such identifications happen after the new environment has been already successfully invaded (Garland & Zimmer 2002).
Fig. 7
Main reports of Limnoperna fortunei in the Paraná Basin (solid grey area). Black triangles represent hydroelectric power plants and blue dots represent water bodies. The red marker inside the red circle represents records from this communication. The red limited area represents the Cerrado biome
In recent years, other authors have been developing detection techniques within molecular biology in order to allow for more efficient detection of the golden mussel in all stages of development (Silva De Paula et al. 2020). These authors underline the importance of prompt detection and fast response at the onset of the invasive process is essential to control the growth (Vander Zanden & Olden 2008) reproduction underlying sex determination (Reis et al. 2023) and preventing further infestations in new environments. This is the case to identify the larvae of bivalve molluscs. Optical identification techniques are visible only at the microscopic level (Garland & Zimmer 2002) as in this present work.
Our results indicate the process of invasion was enhanced by human activities such as fish farming, as the occurrence was registered (Figs 8–12) in and around the structures of the power plant and in fish farm cages upstream the dam, as well as downstream, while there is no transportation or commercial navigation that crosses the river over the dam. Likewise, Barbosa et al. (2016) reported occurrence of the golden mussel near a network of fish cages in the São Francisco River basin, 1,500 km away from the known geographical distribution in South America. Our study adds to the more general evaluation of aquatic invasions in South America in relation to climate change and human activity, most especially in molluscs (Gallardo & Aldridge 2013, Ludwig et al. 2020, Darrigran et al. 2020). It is especially challenging for countries like Brazil that have many rivers, lakes and freshwater reservoirs (Shimizu et al. 2022) where the natural dispersal of species into new environments typically occurs within limited ranges due to geographical barriers increased by human activity (Yang et al. 2023).
Figs 8–12
Limnoperna fortunei of the Paraná: 8 – golden mussel attached all over the fish cage in the mid Paranaíba River fish farm; 9 – incrustation of golden mussel on floating buoy; 10 – golden mussel found in the hatch door to a unit power in the hydroelectric power plant; 11 – golden mussels inside the access to a turbine; 12 – golden mussels attached on the walls of the cooling system pipe

The great density of golden mussels observed in the local fish farm, especially the number of individuals attached to the fishnets (Fig. 8), caused the crane system to break several times due to weight increase of the cages. Even incrustations on the floating buoys compromised harvest (Fig. 9). These fish cages have to be cleaned more often, as the golden mussel incrustations can obliterate water passage, affecting its renewal, and causing injuries to the fish, which in turn depreciates selling price.
The hydroelectric power plant also reported negative impacts on energy production since the power unit generators had to be stopped more often for maintenance. Golden mussels were found attached to several structures inside the power plant (Figs 10–12) and generated expenditures with expensive methods to remove the incrustations from large areas.
Fouling bivalves are also a serious threat to the normal functioning of potable water supply systems, either for human consumption or for industrial use, as they reduce pump efficiency and increase tube corrosion (Darrigran & Ezcurra-De-Drago 2000). The invasion history of the Eurasian zebra mussel (D. polymorpha) in North America demonstrates the potential impacts the Cerrado biome might endure upon the arrival of the golden mussel. In the present scenario, the city water supply needs urgent preventive methods to avoid interruption of the services in the future due to pipes clogged with the golden mussel.
Connelly et al. (2007) estimated 267 million USD in total economic costs for electric generation and water treatment facilities due to fouling through late 2004 in North America (Lake St. Clair). Brazil has 1,267 hydroelectric generation systems, which represent 64.5% of the total power output (ANEEL 2017). Mata (2011) reported that the hydroelectric power plants invaded in Brazil account for almost 10% of energy production and, because of the golden mussel, maintenance and cleaning interruptions were 98.8% more frequent, with costs in mitigation services being over 200,000 USD per year in each one.
The effects of macrofouling processes in hydroelectric power plants and potable water supply systems are beyond economical and can greatly impact the aquatic environment (Boltovskoy et al. 2009, Cataldo et al. 2012, Boltovskoy & Correa 2015, Souza et al. 2023). However, it has not yet been possible to develop an efficient prevention system or any sustainable species management program regarding the golden mussel. Therefore, all the identified dissemination activities must be strictly inspected in order to avoid a broader spread, and the compromising of diversity in other basins or the possible extinction of other freshwater molluscs (Régnier et al. 2009). However, a recent study elaborated by Freitas De Medeiros & Medeiros (2022) investigated the cohabitation of freshwater sponges as a natural competitor of the invasive mussel in the Upper Paraná River. The present study exemplifies an exceptionally rapid reproduction of the golden mussel, and demonstrates the use of histological techniques to determine the maturity of gonads. It also highlights some of the specific conditions under which this species can occur in rivers, lakes and freshwater systems, which in Brazil are abundant (twelve hydrographic basins in the CBEIH 2020).