INTRODUCTION
Invasive pest species cause economic and biodiversity losses (Pejchar & Mooney 2009, Lowry et al. 2013, Pyšek et al. 2020, Fantle-Lepczyk et al. 2022). Cost-effective measures to eradicate or mitigate the negative effects of already established persistent populations are rarely available (Hoffmann et al. 2016, Green & Grosholz 2021). Eradication of invasive species detected at an early stage and preventing the translocation of invasive species, both locally and to new areas, are perhaps the only effective long-term strategies to mitigate the damage caused by biological invasions (Reaser et al. 2020). To develop species-specific strategies, basic ecological knowledge about the invasive species in their new environments is crucial, because behaviour as well as life history characteristics may change due to ecological release (Lambrinos 2004) when transferred from their native area to a new location (Herrmann et al. 2021).
Many terrestrial slugs have the potential to inflict substantial damage to horticultural and agricultural crops (South 1992). Recent introductions of certain species have led to their emergence as serious and invasive pests (Frank 1998, Zając et al. 2017, Barua et al. 2021). Over the last five decades, for example, the large slug Arion vulgaris Moquin-Tandon, 1855 has successfully established invasive populations within anthropogenic landscapes across numerous northern European countries (Zając et al. 2017, 2020, Reise et al. 2020). Similarly, Arion subfuscus s.l. has become established in North America. Conversely, some species, such as Deroceras reticulatum, have been considered pest species in both their native and non-native ranges for a long time (Barker 1991, Willis et al. 2006). A recently spreading slug species is Krynickillus melanocephalus, originating from the Caucasian region and Crimean Mountains (Likharev & Wiktor 1980). It was first found outside of its native location in Ukraine (outside Crimea) in 1998, followed by observations in Germany in 1994, Latvia in 1997, Belarus in 2001, Sweden in 2015, Lithuania, Estonia and Finland in 2017 and Hungary in 2019 (Likharev & Wiktor 1980, Korol & Korniushin 2002, Wiktor & Jurkowska 2007, Šteffek et al. 2008, Stalažs et al. 2017, von Proschwitz 2020, Turóci et al. 2020). Although potential negative effects on crops have not yet been extensively described, instances of damage to pumpkin, strawberries, lettuce and cabbage have been reported (von Proschwitz 2020, Turóci et al. 2020). In Sweden, K. melanocephalus has gained attention from the Swedish Environmental Protection Agency due to its potential invasiveness. However, little is currently known about its basic ecology, such as habitat use, foraging behaviour and interactions with other terrestrial gastropods (Watz & Nyqvist 2022). The primary dispersal vector is human trade in plants and soil. As a result, the species has, so far, been associated with gardens and urban forested areas in its new distribution range (von Proschwitz 2020).
Over the autumn, adults of K. melanocephalus die after laying eggs (von Proschwitz 2020), and the species likely overwinter in the form of eggs (at least at northern latitudes). A crucial factor for developing measures to impede further spread may be to prevent the transportation of soil containing slug eggs. Therefore, having knowledge about the egg-laying substrate preference of K. melanocephalus is critical for offering advice regarding soil transportation; in areas with established populations, the transportation of soils with certain substrates preferred for egg laying should likely be avoided. Furthermore, to predict the potential future northern distribution limit, understanding the tolerance of the eggs to freezing is essential. In the laboratory, we initially investigated the egg-laying substrate preference of K. melanocephalus, and in a subsequent experiment, we tested the winter survival of the eggs by examining their susceptibility to low temperatures.
MATERIAL AND METHODS
EXPERIMENTAL ARENAS
We used five replicated habitat selection arenas. An arena consisted of a closed plastic container (length × width × height = 31 × 23 × 18 cm). On the bottom, four open equally-sized plastic trays (14 × 10 × 8 cm) were placed, creating four quadrants (Fig. 1). The four trays were filled with different substrates: (1) birch leaves (Betula sp.), (2) gravel (diameter = 2–20 mm), (3) potting soil and (4) sphagnum moss (Sphagnum sp.). All substrates were wetted, which created a humid environment inside the containers. Substrate pH (measured in 2 L water mixed with the substrate from one tray) ranged from 5 to 7 (Table 1). Slugs could access the different substrates as well as crawl on the plastic floor, walls and ceiling. In the centre, on top of the trays with contact with all four quadrants, a circular open plastic cup (diameter = 6 cm) functioned as the starting point for slugs in the experiment (Fig. 1). The arenas were kept in a temperature-controlled room at 8 °C with the photoperiod (light:dark) 10:14 h, corresponding to conditions in October in south-central Sweden.
Fig. 1
One of five replicate arenas used for testing egg-laying habitat selection of Krynickillus melanocephalus. Slugs were offered four different substrates (potting soil, birch leaves, gravel and sphagnum moss) to lay their eggs. A slug individual is visible in the circular cup in the centre, where slugs were released at the beginning of the experiment

SLUGS
Specimens of K. melanocephalus were collected in the village of Lagan (56.91°N, 13.99°E) on 24 September 2022, and transported to Karlstad University the day after. Slugs were kept in two 50 L plastic containers provided with humid grass and were fed mushrooms and tomato. We used 60 slugs in the experiments, and at the start of the experiment the mean body mass (± SD) was 0.49 (± 0.17) g. Slugs were not fed during the experiment.
HABITAT PREFERENCE EXPERIMENT
The habitat preference experiment started on 24 October 2022 at 11:00 by placing ten randomly selected slugs in each plastic cup in the centre of the arenas (50 slugs in total). After 24, 48 and 72 h, we recorded the location of the slugs by noting where each individual slug was positioned (i.e., on the plastic floor, wall or ceiling, on top of a substrate or buried inside a substrate). When all slugs had been found and their locations recorded, we placed them in the plastic cup, so that they could select habitat again. Dead slugs (n = 10 during the three days) were replaced with new slugs of similar size (and thus 60 slugs participated in the experiment in total).
EGG DEPOSITION EXPERIMENT
The 50 slugs from the last day of the habitat preference experiment were let undisturbed from 27 October and onwards. On 7 and 14 November (i.e., after 11 and 18 days), we carefully searched the four different substrates for slug eggs and counted the eggs per container and substrate. The egg laying experiment was terminated on 14 November, and the slugs were killed by decapitation.
EGG HATCHING EXPERIMENT
All eggs collected on 7 and 14 November were pooled, kept in wet birch leaves and stored in 4 °C until 3 December, when we distributed 100 eggs randomly between ten small plastic containers (also containing wet birch leaves; 12 × 12 × 5 cm; ten eggs per container). We placed a temperature logger (HOBO Pendant MX Temp/Light, Onset, Bourne, USA) inside each container. Eight containers were put inside polystyrene boxes and placed at different positions in a refrigerator, whereas two containers were placed directly inside the refrigerator (without polystyrene boxes). We chilled the inside of the polystyrene boxes by placing icepacks of different sizes inside them, and icepacks were changed twice every week. Thereby, the eggs were subjected to different winter temperatures (Table 2), with median values that varied between −0.3 and 8.0 °C. When changing the icepacks, we checked the containers to record hatched eggs. On 3 April, we removed the icepacks, and the temperature thereby gradually increased to 8 °C in all containers. The egg hatching experiment was terminated on 5 June. We destroyed eggs that had not hatched and killed slug juveniles by decapitation.
Table 2
Temperature regimes (3 December – 3 April) used in an egg hatching experiment. Eggs of Krynickillus melanocephalus (n = 100) were equally distributed between ten containers and kept in 4 °C until 3 December. The containers were subjected to different temperature treatments from 3 December 2022 to 3 April 2023, after which they all were placed in 8 °C. Containers are listed based on temperature treatment, from the coldest to the warmest temperature regime. Temperatures were recorded hourly
DATA ANALYSIS
We considered a slug using one of the four substrates when the slug had physical contact with the substrate, either by being located on top of the substrate or buried inside it. We used the mean proportion across the three days for each substrate in the arenas as the response variable in our analysis of habitat use and the five arenas as experimental units. Since most slugs were found in contact with plastic (on the floor, walls or ceiling), we considered problems with autocorrelation to be minor. We included substrate as a fixed factor and arena ID as a random factor in a linear mixed model. For the analysis of egg deposition, we used the arenas as replicates in separate one-sample t-tests (one for each substrate) to investigate if the mean proportion of eggs found in respective substrate deviated from the expected 0.25 if eggs were deposited at random.
RESULTS
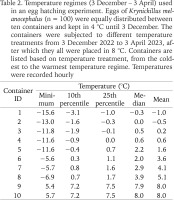
Of the 50 slugs, 18 individuals had contact with a substrate other than plastic when located on the first day of observations, 12 on the second and 17 on the third day. Therefore, the majority of the slugs were observed on the plastic floor, walls or ceiling (which likely relate to the larger plastic surface area than those of the egg-laying substrates). We found more slugs (mean proportion ± SE) in contact with birch leaves (0.17 ± 0.01) than with gravel (0.06 ± 0.03), potting soil (0.06 ± 0.01) and sphagnum moss (0.03 ± 0.01) (Fig. 2). This difference was indicated by a significant effect of substrate in the linear mixed model (F = 11.0; df = 3.16; p < 0.001).
Fig. 2
Proportion of Krynickillus melanocephalus adults (n = 50) located in contact with one of four different substrates at the onset of the egg laying period. Error bars indicate ± 1 SE
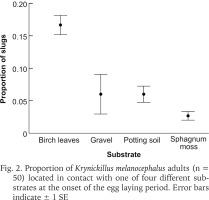
We found 157 eggs in birch leaves, 13 in gravel, seven in potting soil and one egg in sphagnum moss. No eggs were found outside of the four substrates. The mean proportions of eggs (± SE) laid in respective substrate were: birch leaves 0.88 ± 0.06, gravel 0.06 ± 0.02, potting soil 0.05 ± 0.05 and sphagnum moss 0.01 ± 0.01 (Fig. 3). All these mean proportions deviated from 0.25 (t = 10.7, −9.75, −3.821 and −44.0; df = 4; p < 0.01).
Fig. 3
Proportion of Krynickillus melanocephalus eggs deposited in with one of four different substrates. Error bars indicate ± 1 SE and the dashed line the proportion 0.25 that would be expected if eggs were laid at random
The first egg to hatch was found on 27 January in a container with the warmest temperature regime (8 °C; Table 1), and by 14 February, six of the ten eggs had hatched in this container. In the other container held at 8 °C, eggs started to hatch on 7 February, and by 20 February, seven eggs had hatched. No other eggs in these or other containers hatched during the experiment. A newly hatched slug was approximately 5 mm long when extended with a body mass of 5 mg.
DISCUSSION
Previous studies dealing with slugs’ egg laying have been based on observations (e.g., Barker 1991, South 1992). In our study, we investigated habitat preferences experimentally by letting the slugs select habitat when provided equal access to different substrates. We did not check the state of maturity of the slugs, and if some slugs were not mature, we likely underestimated fecundity. Birch leaves were clearly preferred by the slugs as egg-laying substrate. The slugs showed a higher level of contact with the birch leaves compared to other substrates, although most slugs were found resting on the plastic floor, walls and ceiling. The time of observation (11:00) did not likely coincide with the time when the slugs were most active in egg laying, and the plastic surface area was larger than those of the egg-laying substrates. Almost 90% of the eggs discovered were buried within the birch leaves. A notable difference among birch leaves, sphagnum moss, and potting soil was their respective pH. The sphagnum moss and the potting soil were acidic (with a pH of approximately 5), whereas the birch leaves had an almost neutral pH, which may have contributed to the slugs’ preference for the birch leaves. Potentially, leaf composts with low pH (e.g., oak leaves) may not be preferred. On the other hand, pH around 5 does not generally seem to negatively affect slug diversity and abundances (Kappes 2006). The gravel substrate also had a neutral pH, but possibly this substrate had other features that made it unfavoured, such as lack of organic material or its physical structure. An in-depth investigation of which properties of the birch leaves that attracted the slugs lies outside the scope of this study, and mechanisms underlying habitat selection patterns in slugs in general may warrant further investigation (Karlin & Bacon 1961, Willis et al. 2008, Douglas & Tooker 2012).
In Arion vulgaris, approximately 600–700 degree days are needed for egg development (Slotsbo et al. 2013). Eggs may survive subzero temperatures of −2 °C by supercooling, but at lower temperatures eggs freeze and die (Slotsbo et al. 2011). Some slug species are to some extent tolerant to freezing; for example, Deroceras reticulatum, Deroceras laeve and Arion circumscriptus eggs tolerate brief freezing (about 0.5–1 h) with some ice crystal formation (Cook 2004, Storey et al. 2007). In our study, exposure to freezing temperatures (< −5 °C), albeit only for a relatively short while, seemed to be lethal for K. melanocephalus eggs. All eggs that experienced these freezing temperatures died. In containers kept at temperatures above zero (~8 °C), 60–70% of the eggs hatched after approximately three months which corresponded to 640 degree days. Results from future more detailed studies on cold resistance in K. melanocephalus, together with projections of climate change, may be used to model its northern distribution limit (Hatteland et al. 2013).
Whereas almost all eggs were deposited in birch leaves, some eggs were found in all four substrates. Perhaps K. melanocephalus deposit eggs in the most suitable substrate of those that are available, and if there is no leaf compost present (or other substrates with similar properties), they will likely use other substrates. Therefore, transporting any soil, gravel or compost material, as well as plants rooted in such substrates, from areas inhabited by K. melanocephalus could potentially contribute to spreading the species. Leaf composts in these areas warrant extra attention, and it may be advisable to consider incinerating leaf compost material in these areas to prevent further spread. Moreover, plants that are traded from such areas should perhaps be treated with slug control chemicals by fumigation (South 1992) or banned completely.


