INTRODUCTION
The Cyclophoroidea, with 3,671 species, is the second largest group of terrestrial snails after Stylommatophora, which contains 22,356 species (MolluscaBase eds. 2023). Similar to their aquatic relatives, cyclophoroidean snails possess an operculum, which is a corneous or calcareous trapdoor-like apparatus attached to the upper surface of their posterior foot (Ponder et al. 2019: 96). When the cyclophoroidean snail withdraws its body to its shell, the operculum seals the aperture so tightly, that probably no gas exchange becomes possible. To overcome this problem, several groups have developed by-pass channels formed by internal plicae (Páll-Gergely et al. 2015), canals and tubes (e.g., Rees 1964, Páll-Gergely et al. 2014, 2017, Jirapatrasilp et al. 2022, Tongkerd et al. 2023), to allow gas exchange even when the operculum seals the aperture. The breathing tubes are open at the outer end in all groups, except for the Asian Alycaeidae, in which it is closed (Fig. 1). Since it is not self-evident how a closed tube is useful for gas exchange, this phenomenon posed a century-old mystery (Godwin-Austen 1882–1920, Rees 1964). This was solved by the discovery of microtunnels that run beneath radial ribs along the growth line of the last whorl and open into the main breathing tube. These tunnels, formed by the outermost shell layers, open to the outside of the shell surface near the umbilicus, and probably allow gas exchange with a closed operculum (Páll-Gergely et al. 2016, Fig. 1).
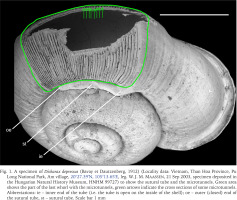
Fig. 1
A specimen of Dicharax depressus (Bavay et Dautzenberg, 1912) (Locality data: Vietnam, Than Hoa Province, Pu Long National Park, Am village, 20°27.39′N, 105°13.65′E, leg. W. J. M. Maassen, 21 Sep 2003, specimen deposited in the Hungarian Natural History Museum, HNHM 99727) to show the sutural tube and the microtunnels. Green area shows the part of the last whorl with the microtunnels, green arrows indicate the cross sections of some microtunnels. Abbreviations: ie – inner end of the tube (i.e. the tube is open on the inside of the shell); oe – outer (closed) end of the sutural tube, st – sutural tube. Scale bar 1 mm
The presence of the sutural tubes in the Alycaeidae raises a question on its ontogeny and development. Specifically, whether they are created as the shell grows and forms the microtunnels, or whether the tube is formed after all the microtunnels have been established.
A note on the tube formation were published in a hard-to-reach Japanese journal by Matsumoto (1993). Here I provide a translation by J. U. Otani: “Although I had thought the breathing tube of alycaeids were made along with growth and at the same time as the whorl tube, an observation of a juvenile shell collected presently makes it clearly obvious that the whorl tube is once made up to a position close to the aperture, and when the position at which the breathing tube is to be made is approached, the portions of the growth ribs close to the suture become formed so as to be distorted in the direction opposite to the direction of growth. The portion at which the breathing tube is to be attached is formed in a slightly recessed manner and then gradually increases in curvature, and when a certain position is reached, the breathing tube becomes formed in the opposite direction along the suture from that position”.
In this paper, I confirm this observation with a teratological specimen of Dicharax robustus Páll-Gergely et Hunyadi, 2017 and a subadult specimen of Dicharax cf. strangulatus (L. Pfeiffer, 1846), and prove that the alycaeid tube is built “backwards”.
MATERIAL AND METHODS
During the examination of several thousand alycaeid shells in private and public collections, I found only two shells to provide information on the ontogeny of the sutural tube. The first specimen is a teratological specimen of Dicharax robustus Páll-Gergely et Hunyadi, 2017 (China, Yunnan, Kunming Shi, Yuqiqu, Bijianshan, Guanyinsi (temple), approximate GPS data: 24°16′16″N, 102°49′44″E, leg. K. Ohara, 19.03.2000, collection K. Ohara, see Figs 2–5). The second one is a subadult of Dicharax cf. strangulatus (L. Pfeiffer, 1846) (Nepal, Prov. Seti, Distr. Bajhang, Shima, Ghatganga Khola NE Shima, Ahorn-Kastanien-Eichen-Blockschuttwald, 29°45′08″N, 81°23′30″E, leg. U. Bössneck, 25.06.2009, HNHM 105552) (Figs 6–8).
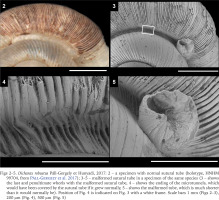
Figs 2–5
Dicharax robustus Páll-Gergely et Hunyadi, 2017: 2 – a specimen with normal sutural tube (holotype, HNHM 99704, from Páll-Gergely et al. 2017); 3–5 – malformed sutural tube in a specimen of the same species (3 – shows the last and penultimate whorls with the malformed sutural tube, 4 – shows the ending of the microtunnels, which would have been covered by the sutural tube if it grew normally, 5 – shows the malformed tube, which is much shorter than it would normally be). Position of Fig. 4 is indicated on Fig. 3 with a white frame. Scale bars 1 mm (Figs 2–3), 200 µm (Fig. 4), 500 µm (Fig. 5)
Figs 6–8
Adult (6) and subadult (7–8) shells of Dicharax cf. strangulatus (L. Pfeiffer, 1846). Red line indicates the shell part which contains the microtunnels (termed R2 in Páll-Gergely et al. 2017). Dashed line indicates the approximate outline of the subadult shell if it was adult. White line in Fig. 8 shows the endings of microtunnels that are not yet covered by the sutural tube. Green arrow on Fig. 6 shows the presence, and on Fig. 7 the absence of the sutural tube. Scale bars 1 mm

Shells were directly observed without coating under a low vacuum SEM (Miniscope TM-1000, Hitachi High-Technologies, Tokyo).
RESULTS AND DISCUSSION
According to the observations of Matsumoto (1993), the alycaeid tube is formed “backwards”, after the microtunnels are made (although the presence of the microtunnels were unknown at that time). In the teratological Dicharax robustus specimen, the microtunnels are clearly visible (Figs 3–4), but the tube formation stopped for an unknown reason, and the tube is incompletely developed, short, and slightly pointing upwards (Fig. 5). In the subadult specimen of Dicharax cf. strangulatus, the endings of the microtunnels are clearly visible near the suture (see Fig. 8), but no sutural tube is formed yet (Figs 7–8). In contrast, the adult specimen has a sutural tube in the position (Fig. 6) where the subadult shell has no tube yet. Both observations agree with that of Matsumoto (1993). These results suggest that microtunnels may be functional without an outer tube in other groups of terrestrial Caenogastropoda. It remains to be investigated whether the tube formation is different between species having short and long (up to a half whorl, i.e. Alycaeus Gray, 1850, see Páll-Gergely 2023) sutural tubes.

