INTRODUCTION
India has a coastline of more than seven thousand kilometres and the heterogeneity of the eco-geography from this long coastal stretch has given a rich species diversity. The state of West Bengal alone covers a 157 km long coastline among the northern part of Bay of Bengal and it is one of the biogeography hotspots, Sunderban Biosphere Reserve (SBR) is a crown jewel for its species richness in this coastal estuarine region (Danda et al. 2017). The oldest and largest existing single mangrove belt is Sunderban, recognised as one of the most productive ecosystems of the world covering an area of 4,264 km2 in the Indian region. The confluence of river Brahmaputra and Ganga gave rise to numerous islands, creeks and riverine channels, thus forming the mangrove network of Sunderban Biosphere Reserve. Following the immense importance of this mangrove ecosystem, United Nations Educational, Scientific, and Cultural Organization (UNESCO) declared the Indian portion of Sunderban a World Heritage Site since 1987 and SBR has been designated its status as a biosphere reserve under the UNESCO Man and the Biosphere program in 2001. The diverse habitat of Sunderban encompasses a diverse landscape, from mudflats, sandy and muddy beaches, small estuarine channels to vast intertidal mangrove belt (Chandra et al. 2017, FSI 2017).
Molluscs are one of the most important group of invertebrates in terms of species richness, biomass and abundance covering almost every possible habitat except for the aerial (Kabir et al. 2014). Mangroves are one of the most productive ecosystems harbouring a large diversity of molluscs, and their species composition varies with several factors of the ecosystem such as exploitation, disturbance, and rehabilitation of mangrove trees (for the protection of mangrove belt along the coastline). Molluscs can thus be used as a major indicator to assess the ecosystem health (Skilleter & Warren 2000). In-terms of food chain roles and ecosystem services, molluscs are a major source of food for several birds and fishes; they act in conversion of organic detritus, which is one of the bases for carbon sequestration in a mangrove ecosystem. The algal component of estuaries, which is a major storage of carbon, forms part of carbon sink and carbon chain with the help of the molluscs feeding on these algal components (Mitra et al. 2013). Several factors like habitat and shore line alteration, uncontrolled exploitation of natural resources, commercialisation of shells, pollution from various sources, are acting as destroyers of malacofaunal diversity of Sunderban, which could be a major factor in future for the survivability of Sunderban Biosphere Reserve (Danda et al. 2017).
Research on the molluscs of lower Bengal started with the work of Stoliczka (1869), followed by study of brackish water molluscs of Nevill (1880), and later contributions by Annandale (1907, 1922) laid the foundation for the study of molluscan fauna in this area. Presently 5,249 species of Mollusca have been reported from the Indian Subcontinent and a total of 301 species has been recorded from the coastal and estuarine part of the state of West Bengal (Tudu et al. 2018a, Banerjee et al. 2022). The last updated work from SBR by Zoological Survey of India and World Wildlife fund in 2017, recorded 177 molluscan species from this region (Chandra et al. 2017, Danda et al. 2017). Thus, this present work gives an account of five new molluscan records from SBR; three new records from the state of West Bengal and one new record from India.
MATERIAL AND METHODS
A number of surveys were undertaken for exploring the malacofaunal diversity of Sunderban Biosphere Reserve (SBR). Molluscan samples were gathered from various locations, including Dhanchi Island (21°37'43.96"N, 88°26'48.31"E), Jamboo Island (21°34'39.56"N, 88°11'33.83"E), Lothian Wildlife Sanctuary (21°36'31.91"N, 88°18'50.65"E), Sagar Island (21°38'21.56"N, 88°4'44.86"E), Mousuni Island (21°37'44.34"N, 88°11'58.95"E), Kakdwip harbor (21°51'35.13"N, 88°10'41.06"E), and Bakkhali (21°33'27.71"N, 88°16'29.71"E). Among these, Dhanchi, Jamboo, and Lothian are situated within the protected area of the SBR (Sunderban Reserve), while the other locations are tourist attractions situated in the transitional zone of the SBR. Except for Kakdwip and Bakkhali, these locations are isolated from the mainland and are all situated in the southwestern part of the Indian Sunderban. They are characterised by sandy beaches in the southern region, extensive open mudflats, and thriving mangrove vegetation (Figs 1–2).
Figs 1–2
Map showing the collection localities of newly recorded molluscan species from Sunderban Biosphere Reserve. 1 – Map of the Sunderban Biosphere Reserve, 2 – Zoomed portion of the collection localities: A – Kakdwip (collection site for B. spirata, B. zeylanica), B – Sagar Island (collection site for B. spirata), C – Mousuni Island (collection site for A. perdix), D – Jamboo Island (collection site for I. gradata, A. perdix), E – Bakkhali (collection site for A. perdix), F – Lothian Wildlife Sanctuaruy (collection site for A. perdix), G – Dhanchi Island (collection site for D. tumida)

After collection, live specimens were fixed in 10% formalin (in river/marine water) solution and later preserved in 70% ethanol for long term preservation. Shells were washed in fresh water after collection and dried in the sun. Identification and classification used here is based on Rao (2003, 2017) and WoRMS (http://www.marinespecies.org/). All length measurements were taken to nearest 0.1 mm using electronic callipers. Specimens are deposited in the National Zoological Collections of the Sunderban Regional Centre of the Zoological Survey of India.
RESULTS
The details of the new species for Sunderban Biosphere Reserve (SBR) are given below:
Class Bivalvia Linnaeus, 1758
Subclass Autobranchia Grobben, 1894
Order Venerida Gray, 1854
Family Veneridae Rafinesque, 1815
Genus Dosinia Scopoli, 1777
Dosinia tumida (Gray, 1838)
Arthemis tumida Gray 1838
Conservation Status Not Evaluated (IUCN 2022).
Material examined: ZSI/SbRC/KN6455, 2 samples, total length (53–60 mm), Dhanchi Island, Sunderban Biosphere Reserve (21°37'43.96"N, 88°26'48.31"E), 12 March 2020, Coll: S. Banerjee.
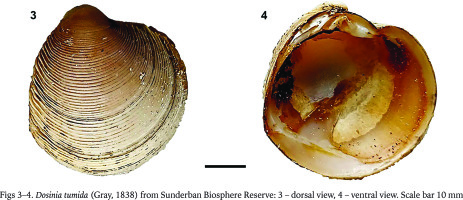
Description. Shell is properly orbicular and maximum distance between ventral ligament and escutcheon is 5 mm. Escutcheon is narrow and its shell is smooth. Umbo is prominent and obliquely erected to the anterior end. Lunule is black coloured and shaped like a heart. Internal part of the shell is smooth and the muscle scar is almost the same size as the shell (Figs 3–4).
Figs 3–4
Dosinia tumida (Gray, 1838) from Sunderban Biosphere Reserve: 3 – dorsal view, 4 – ventral view. Scale bar 10 mm
Habitat. Only two samples were collected from the intertidal zone of a sandy beach near an estuarine zone. This beach is protected area of Sunderban and no human interference is observed in the area. The area of the island nearby is protected with mangrove vegetation.
Distribution. India – Orissa, Karnataka (Boominathan et al. 2012, Tudu et al. 2018b); Elsewhere – Indo-West Pacific: Qatar, Saudi Arabia and Indonesia (Palomares & Pauly 2022).
Remarks. As per Tenjing et al. (2019), seven species of Dosinia were reported from India, namely D. bruguieri, D. cretacea, D. fibula, D. histrio, D. prostrate, D. tranquebarica. Tudu et al. (2018a) recorded a single species of Dosinia, namely D. areolata from the Digha coast of West Bengal. Thus, this is the first record of D. tumida from Sunderban Biosphere Reserve and the state of West Bengal. D. areolate has a lanceolate ovate (egg) shaped lunule with a small and sharp umbo, which is hardly protruding whereas D. tumida does not have these characters. Escutcheon of D. areolate is wider than D. tumida. The discovery of D. tumida on Dhanchi island demonstrates the existence of Dosinia in the Sunderban, but because the collection site is not a human habituated island and there have been relatively few surveys, it is possible that this has led to the species’ neglect in the region.
Class Gastropoda Cuvier, 1795
Subclass Caenogastropoda Cox, 1960
Order Neogastropoda Wenz, 1938
Family Babyloniidae Kuroda, Habe & Oyama, 1971
Genus Babylonia Schlüter, 1838
Babylonia spirata (Linnaeus, 1758)
Buccinum spiratum Linnaeus 1758
Conservation Status: Not Evaluated (IUCN 2022).
Material examined: ZSI/SbRC/KN6366, 1 specimen, total length (26.04 mm), Ganga Sagar, Sunderban Biosphere Reserve (21°38'21.56"N, 88°04'44.86"E), 20 August 2021, Coll: J. S. Yogesh Kumar & Co. ZSI/SbRC/KN5150, 3 specimens, total length (26.12 mm, 27.64 mm, 28.37 mm), Kakdwip Harbour, Sunderban Biosphere Reserve (21°51'35.13"N, 88°10'41.06"E), 06 December 2019, Coll: A. Sen.
Description. Specimen with a broad and ovately conical shell, shorter than in other Babylonia sp. Spire with a prominent and pointed end. Sutural canal is prominently wide and deep, visible with an angular keel shaped shoulder. Body whorl overall rounded but moderately flattened between shoulder and upper periphery. Shell aperture dimension is half of shell height. Periostracum is thin and brownish coloured. Umbilicus is wide open. Base shell colour is off-white with dark brown blotches arranged over the shell in spiral bands. Size and shape of these blotches are variable in nature (Figs 5–6).
Figs 5–10
Molluscs collected from Sunderban Biosphere Reserve: 5–6 – Babylonia spirata (Linnaeus, 1758), dorsal and ventral view, 7–8 – Babylonia zeylanica (Bruguière, 1789), dorsal and ventral view, 9–10 – Indothais gradata (Jonas, 1846), dorsal and ventral view. Scale bars 10 mm

Distribution. India – Tamil Nadu, Kerala, Orissa, Gujrat, Maharashtra, Goa, Karnataka, Andhrapradesh, West Bengal (Mohan 2007, Chelladurai et al. 2013, Tudu et al. 2018a, Chandra et al. 2020); Elsewhere – Indo-West Pacific (Palomares & Pauly 2022).
Habitat. One shell sample was collected from the intertidal sandy beaches of Sagar Island, which is a mostly estuarine site. Three live specimens were collected from a fishing boat near Kakdwip harbor. This fishing boat was fishing inside the estuarine region of Matla River. The fishermen regarded them as trash.
Remarks. B. spirata has a wide distribution around the Indian coast line and this species is widely popular for its usage in preparation of cultural artifacts and shell ornaments. This species is recorded in the state of West Bengal only in a single document and its specific collection locality is not known (Chandra et al. 2020). The species was never reported from the SBR and thus this record provides the first proof of this species from SBR (Chandra et al. 2017, Tudu & Balakrishnan 2018).
Babylonia zeylanica (Bruguière, 1789)
Buccinum zeylanicumBruguière1789 (Original description not documented).
Buccinum giratumRöding1798.
Conservation Status: Not Evaluated (IUCN 2022).
Material examined: ZSI/SbRC/KN5149, 1 specimen, total length (39.2 mm), Ganga Sagar, Sunderban Biosphere Reserve (21°37'52.48"N, 88°06'57.13"E), 21 August 2021, Coll: J. S. Yogesh Kumar & Co.
Description. Shell is conical with a slender shape and total length is 57 mm. Body whorls – 7. Shell aperture is 27 mm, almost half of the total length. Whorls are more inflated and shell is somewhat thinner than B. spirata. Sutures are not canaliculated. Shoulder does not have a proper structure. Outer lip of the aperture is slightly thickened and sharp at the upper part. Inner lip callus with an umbilical notch. Umbilicus with a broad opening and teeth placed at outer margin. Periostracum thin and coloured slightly yellowish. Shell base colour is off-white with light to deep brown blotches over it, in a linear formation with the lowest spot of the body whorl (Figs 7–8).
Distribution. India – Tamil Nadu, Kerala, Orissa, Andhra Pradesh, Pondicherry, Goa, Gujrat (Mohan 2007, Chelladurai et al. 2013, Tudu et al. 2018b, Chandra et al. 2020), Elsewhere – Indonesia, Sri Lanka, Taiwan, New Zealand, Australia (GBIF 2022a).
Habitat. One shell sample was collected from the intertidal sandy beaches of Sagar Island, which is a mostly estuarine site. B. zeylanica is termed as Indian Babylonia and it was thought that this species only had a distribution from the western Indian Ocean (Mohan 2007). Although this present study and previously documented data provides a clear distribution pattern of this species along the complete Indian coastline.
Remarks. Previously the species was recorded from Orissa coast (Tudu et al. 2018b). However, it had not been observed in the northern part of the Bay of Bengal (Chandra et al. 2017, Tudu & Balakrishnan 2018). The present study marks the initial documentation of the species in West Bengal and within the Sunderban Biosphere Reserve. While this species is quite popular in Indian fisheries trade, with both Babylonia species being exported to Thailand, Singapore and Malaysia, it is also increasingly sold in India as whelk meat (Mohan 2007). Both of the Babylonia species are quite common along India’s eastern coast, and these shells are widely used in the region to make shell ornaments. Locals used to gather Babylonia species for this purpose on sandy beaches along the east coast, but these new records of B. spirata and B. zeylanica from Sunderban may reflect its low frequency of occurrence as it is an estuarine site.
Family Muricidae Rafinesque, 1815
Genus Indothais Claremont, Vermeij, S. T. Williams et D. Reid, 2013
Indothais gradata (Jonas, 1846)
Purpura (Cuma) gradata Jonas 1846 (Original description not documented)
Purpura trigona Reeve 1846
Conservation Status: Not Evaluated (IUCN 2022).
Material examined: ZSI/SbRC/KN6023, 3 specimens, total length (26.3 mm, 24.2 mm, 27.1 mm), Jamboo Island, Sunderban Biosphere Reserve (21°34'39.56"N, 88°11'33.83"E), 29 December 2019, Coll: A. Sen.
Description. The identified shells of Indothais gradata are highly variable in size and shape. The carina has a projection. The structure which separates the last whorl from the others is just below the carina of the second last whorl. The side of the last whorl is angled directly from the carina towards the anterior canal (30 degrees), creating a triangular-shaped aperture. Bottom of surface whorl is smooth. Spire convex shaped with small thorny ridges. Shell is slightly dark and characterised by the colour of the greyish internal aperture. Outer lip is thick and rounded. Siphonal tube is well-developed. Shell colouration is brownish white (Figs 9–10).
Distribution. India – Maharashtra (questionable occurrence), Elsewhere – Thailand, Japan, Singapore, Malaysia, Japan, Vietnam, Australia (GBIF 2022b).
Habitat. A live specimen has been collected from the southern part of Jamboo Island, which is in core area of SBR. No human activities are allowed in this region. The specimen was collected from a mudflat, the southern-side of the area is Bay of Bengal and mangrove vegetation covers the northern part of the area. The species was collected with a sample of Indothais lacera (Born, 1778), which has a wide distribution in SBR.
Remarks. Globally 12 species are recorded under the Indothais genus, but only two were known from the Indian region, I. lacera and I. blanfordi (Melvill, 1893). Both the species are reported along the whole coastline of India (Chandra et al. 2020). Both of these species are widely distributed in SBR (Chandra et al. 2017, Tudu & Balakrishnan 2018). I. lacera can be easily distinguished from other two species by the presence of strongly elevated shouldered body whorls, which is angulated without any elevation for other two. The colouration patterns of I. gradata and I. lacera are totally different. I. gradata has longitudinal rows of brownish bands over the whole shell whereas I. lacera has fine brownish vertical lines arranged in pair over the sculpture. I. gradata shell is quite thin and arrangement of denticles on the outer lip is inconspicuous compared to the other two. Studying the global occurrence pattern of I. gradata revealed that, this species has been mostly observed from the eastern, southeastern Indian Ocean and eastern Pacific Ocean. A single record of I. gradata has been registered in GBIF database from western Indian Ocean from Mumbai, India and from Bangladesh. The collection of the species is mentioned by the US Academy of Natural Sciences, and the Australian Museum. Other collection details and timing are not registered for the Indian specimen and as for the GBIF information source, the details of these collection has been flagged with questionable collection locality and institution details (“Continent derived from coordinates”, “Collection match fuzzy”, “Institution match fuzzy”). Therefore this distribution data is questionable and as the survey of Indian literature on Mollusca conveys, there is no such record of this species from the Indian territory as well as from east coast of India (Chandra et al. 2020). This study provides the first good evidence of the occurrence of I. gradata from India, as well as from SBR. I. gradata might be found in the other estuarine sites of the eastern Indian coast; the absence of records may reflect its occupation of muddy habitats, obscuring its specific features.
Subclass Heterobranchia Burmeister, 1837
Family Architectonicidae Gray, 1850
Genus Architectonica Röding, 1798
Architectonica perdix (Hinds, 1844)
Solarium perdix Hinds 1844
Conservation Status: Not Evaluated (IUCN 2022).
Material examined: ZSI/SbRC/KN5407, 1 specimen, diameter (22.1 mm), Jamboo Island, Sunderban Biosphere Reserve (21°34'39.56"N, 88°11'33.83"E), 08 February 2020, Coll: A. Sen; ZSI/SbRC/KN5803, 1 specimen, diameter (24.3mm), Bakkhali, Sunderban Biosphere Reserve (21°33'27.71"N, 88°16'29.71"E), 10 June 2021, Coll: J. S. Yogesh Kumar; ZSI/SbRC/KN7041, 1 specimen, diameter (22.5 mm), Lothian Wildlife Sanctuary, Sunderban Biosphere Reserve (21°36'31.91"N, 88°18'50.65"E), 12 October 2020, Coll: A. Sen; ZSI/SbRC/KN2808, 1 specimen, diameter (20.7 mm), Mousuni Island, Sunderban Biosphere Reserve (21°37'44.34"N, 88°11'58.95"E), 26 November 2019, Coll: A. Sen; ZSI/SbRC/KN6456, 1 specimen, diameter (19.2 mm), Jamboo Island, Sunderban Biosphere Reserve (21°34'39.56"N, 88°11'33.83"E), 12 March 2020, Coll: S. Banerjee.
Description. Shell is moderately large and shell wall is thick. Overall conical shaped shell, depressed, with inflated whorls at the upper portion. Axial growth lines at the upper portion are finely spaced with very small spaces in between. Absence of additional spiral ribs at periphery. Whorl attachment is margined with the edge of lower peripheral ribs. Absence of spiral ribs at the basal region; two distinct spiral ribs form a nodular ribbon around the umbilicus. Inner lip has a straight margin and umbilical crenae area hanging outside umbilicus. Base body colouration is off-white with a regular and distinctive light to dark brown pattern on the shell. A light brown band is present on the midrib area. Some light brown blotches are present over the basal area (Figs 11–13).
Figs 11–13
Architectonica perdix (Hinds, 1844) from Sunderban Biosphere Reserve: dorsal, lateral, and ventral view). Scale bar 10 mm

Distribution. India – West Bengal (Digha), Pondicherry, Tamil Nadu (Bieler 1993, Dey 2016, Tudu & Balakrishnan 2018), Elsewhere – Eastern Indian Ocean and western to central Pacific Ocean (Tudu & Balakrishnan 2018).
Habitat. Sample shells of A. perdix have been collected from several areas in SBR. The collection locality in Dhanchi Island and Mousuni Island is a mud flat area closely associated with mangrove vegetation. In Jamboo island collection locality is a muddy beach side and in Bakkhali, the locality is a sandy beach site.
Remarks. Previously, this species was recorded from Digha beach of West Bengal, which is typically a sandy beach (Tudu & Balakrishnan 2018). Only one species was reported from Sunderban, that is A. perspectiva (Linnaeus, 1755) (Rao et al. 1992). This present record confirms the presence of A. perdix from Sunderban. A. perdix can be differentiated by the presence of inflated whorls, brown blotches at the base, upper ridge with separate light brown blotches; whereas A. perspectiva has axially incised whorls, fine brown dots at the base, upper ridge with deep brown square blotches appearing almost in a band pattern.
DISCUSSION AND CONCLUSION
The vast island network of Sunderban lies at the northernmost part of Bay of Bengal and SBR has always been a focus for biodiversity research and conservation due to the present threats and never-ending erosion of the mangrove ecosystem. Studies of malacofaunal diversity are vital in the quest to understand the mechanisms of different ecological processes in mangrove based estuarine ecosystems. While there are records from the late 18th century onwards, it is remarkable that more intensive surveys between 1900 and 2022 have revealed the presence of only 177 species within the SBR, considering that more than 5,000 species of Mollusca are recorded from India as a whole (Tudu et al. 2018a, 2018b, Banerjee et al. 2022).
It follows that molluscs in the Sunderban are seriously under-recorded. The inaccessibility of the core area, and a focus on common and easily recognised species no doubt account for this in part. The records reported here come from core areas of the SBR, from mud flats, sandy beaches, and from trawl netting in estuaries. Much effort and long-term studies would be needed to establish the true extent of molluscan diversity in the region. It is important here to emphasise the key role played by molluscs in maintaining ecosystem health, a role that cannot be filled by other organisms. Molluscs store and release calcium, essential for vertebrate bone formation; they are also important elements in the carbon cycle. Bivalves, capable of tolerating a wide range of salinities, can control the abundance of plankton, and molluscs more generally, can accumulate various pollutants that are indicators of ecosystem health (Rumisha et al. 2012). The microhabitat requirements of some species mean that their loss could impact a whole community (Danda et al. 2017).
The lack of comprehensive knowledge thus prevents well-considered conservation measures. Uncommon species, of low mobility and highly specific requirements act as essential bioindicators of change or ecosystem function. We hope that this study, revealing five species previously unknown in the Sunderban, will act as a catalyst for further work to reveal the true molluscan diversity of the region, and enable conservation strategies to have a more secure basis. This is urgently needed.

