INTRODUCTION
Castles, and particularly castle ruins, often combine many types of habitat that rarely occur together naturally, and they can constitute hotspots of biodiversity, sometimes as refugia for rare and endangered species (Juřičková 2005). They have an ecological as well as a cultural significance. They have been frequently the subject of molluscan research in central Europe; in the Czech Republic (Ložek 1959, Brabenec 1954, Juřičková 2005), in Germany (Zeissler 1968, 1975, 1980, Haldemann 1990), and in Poland, where the Carpathian castles were thoroughly examined by Alexandrowicz (1995), who determined the malacocoenoses of 20 castles and castle ruins, including those in Barwałd, Cieszyn, Czchów, Dąbrówka Starzeńska, Dobczyce, Kobiernice, Krajowice, Lanckorona, Monasterzec, Muszyna, Myślenice, Melsztyn, Nowy Sącz, Odrzykoń, Rytro, Ślemień, Rożnów, Tarnów, Wytrzyszczka and Zagórze. There are also studies from the Smoleń castle (Alexandrowicz & Alexandrowicz 2014) and the Lipowiec castle (Alexandrowicz & Alexandrowicz 2017), which constitute a part of a large castle system called “Eagles’ Nests” (Polish: Orle Gniazda), located in the Kraków-Częstochowa Upland. More studies were conducted in the Małopolskie voivodeship, including the Melsztyn castle (Alexandrowicz 2013) and the Wawel Hill in Kraków (Alexandrowicz 1988, Gołas-Siarzewska 2013).
There is less information about the castles in Lower Silesia, south-western Poland. The region is not as rich in limestone as the areas of the Kraków-Częstochowa Upland. However, because calcium carbonate was used in their construction, there is often local enrichment of the soil (Alexandrowicz & Alexandrowicz 2014), and these ruins provide habitats for calciphile species. Studies have been undertaken at Rogowiec castle (Maltz et al. 2019), located in the northern part of the Suche Mountains, a part of the Central Sudetes, and at the castles of Książ, Stary Książ, Cisy, Grodno and Nowy Dwór, located in the glen Kotlina Wałbrzyska and highlands Pogórze Wałbrzyskie (Maltz 1999). A broader study of malacofauna in the Kaczawskie Mountains and Foothills also covered Świny and Bolków castles (Pokryszko 1984). There are also earlier studies by German authors, relating to Silesia or the Sudetes, that sometimes refer to the fauna of castles, such as Bolków castle (Scholtz 1843, Reinhardt 1874, Merkel 1894, Sprick 1921), Cisy castle (Reinhardt 1874, Merkel 1894), Grodno castle (Merkel 1894) and ruins of Homole castle (Sprick 1921). Most of the listed malacological studies were carried out long ago and only once.
It is only rarely that a castle fauna is sampled more than once, for example, that of Wawel Hill (Alexandrowicz 1988, Gołas-Siarzewska 2013). As noted by many authors, repeat sampling of castle malacofaunas is an excellent way to monitor both local and regional change. Molluscs are good indicators of environmental changes, including anthropogenic influence (Juřičková 2005), as they are very numerous and common, have different habitat preferences depending on the species and show limited mobility. Some papers emphasise the great importance of such research not only at the individual species level but also within species groups (Juřičková 2005, Alexandrowicz & Alexandrowicz 2011).
In this study, we report on repeated sampling of the malacofauna of Bolków castle, for which there are records spanning more than a century. For the first time, though, we have used quantitative methods that enable us to examine changes in abundance as well as occurrence, and to test the completeness of the sampling regime.
DESCRIPTION OF STUDY AREA
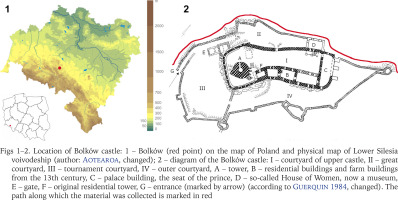
The castle is located in Bolków, a small town in south-western Poland, in the Jawor district in the Lower Silesian voivodeship, on the border of the Western and Central Sudetes, adjoining the Kaczawskie Mountains (Fig. 1). The area is characterised by a transitional climate between foothill and lowland, due to the geographical location and geological structure (Pokryszko 1984). Bolków is located in the Nysa Szalona River valley and the Rochowicka Woda stream connected to it.
Figs 1–2
Location of Bolków castle: 1 – Bolków (red point) on the map of Poland and physical map of Lower Silesia voivodeship (author: Aotearoa, changed); 2 – diagram of the Bolków castle: I – courtyard of upper castle, II – great courtyard, III – tournament courtyard, IV – outer courtyard, A – tower, B – residential buildings and farm buildings from the 13th century, C – palace building, the seat of the prince, D – so-called House of Women, now a museum, E – gate, F – original residential tower, G – entrance (marked by arrow) (according to Guerquin 1984, changed). The path along which the material was collected is marked in red
The castle is built on the highest hill (396 m a.s.l.) in the town. The hill is made of greenstones, greenschists, chlorite and sericite slates, crystalline limestones and some diabases (Żukow-Karczewski 1996). The castle was built using the surrounding raw materials, such as the aforementioned shale and sandstone. The hill is surrounded by deciduous woodland, interspersed with small buildings. Although near the town centre, the castle was, until recently, overgrown with succulents, vines, mosses and lichens. It was, however, extensively renovated in 2015, reducing the cover.
The earliest records of this castle date back to the 13th century. For years it served as a treasury for wealthy princes of the Piast Dynasty. Afterwards, it changed the owner and the function many times, which was the reason for its frequent reconstruction (Żukow-Karczewski 1996). Large fragments of the fortress have survived to the present day, including the walls and the characteristic tower in the shape of a “tear”, 25 metres high. Such a non-standard construction increased its resistance to projectiles. Very few towers in Europe were constructed in this manner (Żukow-Karczewski 1996). The castle was eventually abandoned nearly two centuries ago, and fell into ruin. It is now a tourist attraction, and an important part of the cultural heritage of the region. In addition, festivities and annual festivals are held there, including the popular “Castle Party”, which is one of European largest gothic rock culture events. Therefore, it is a lively attraction in Lower Silesia.
MATERIAL AND METHODS
Surveys were made on three occasions, in 2006, 2010 and 2021. In 2006, sampling was restricted to sampling by eye in the field (leg. T. Maltz, 22.08.2006), while in 2010 (leg. T. Maltz, M. Proćków, E. Kuźnik-Kowalska, 03.09.2010) and 2021 (leg. A. Maculewicz, M. Proćków, E. Kuźnik-Kowalska, 29.05.2021), these searches were supplemented with samples from litter and rock debris, and by material gathered from rock shelves and walls.
All specimens and samples were collected along the walls from the outside, on the left side of the entrance (Fig. 2). There is a path on one side, bordered by the outer part of the walls and the rocks on which the castle is built, while on the other side of the path, there is a downwards slope. It is densely overgrown with vegetation in the form of shrubs, deciduous trees such as ash, sycamore and linden, and tall herbaceous plants.
The manual collection was made along the entire length of the path (Fig. 2). Specimens that could be identified in the field were recorded and returned. Live specimens needing further study were preserved in 70% ethanol. The litter sample was made, following Alexandrowicz & Alexandrowicz (2011), by amalgamating samples from ten randomly selected points along the path. A total volume of 15 litres was passed through a 10 × 10 mm mesh sieve. Rock debris was not sieved. Vegetation and soil were sampled from rock shelves accessible from the path (Figs 3–5). Material from both samples was dried and sieved down to a mesh size of 2 × 2 mm.
Figs 3–5
Bolków castle habitats: 3–4 – walls before renovation (photo: Tomasz K. Maltz, 03.09.2010) (3 – vegetation growing along walls and path, 4 – rock shelves overgrown with succulents, mosses and lichens); 5 – walls after renovation (photo: Elżbieta Kuźnik-Kowalska, 29.05.2021)

Specimens were identified to species where possible, and counted (Table 1), otherwise to family. Nomenclature follows MolluscaBase (2022). All retained material is held in the Museum of Natural History of Wrocław. Each species was assigned to an ecological group (E), following Riedel (1988) and Alexandrowicz & Alexandrowicz (2011). Five ecological groups were recognised: F – shade-loving species, B – species preferring partially shaded habitats, M – mesophilous species tolerating varying degrees of shading, O – open-country species, X – xerophilous species. The quantitative data were presented in the form of classes, divided by the number of specimens found: I (1–3), II (4–10), III (11–31), IV (32–62), V (63–99), VI (100–170). The years of collection and sampling methods are also distinguished in Table 1. In the case of manual collection and including old data (Scholtz 1843, Reinhardt 1874, Merkel 1894, Sprick 1921, Pokryszko 1984), abundance classes were not specified, only the occurrence of individual species was recorded with a symbol “+”. Calculations of the completeness were made for data from 2010 and 2021 using the Chao index (SChao1) (Nichols & Conroy 1996). The dominance index (D) was calculated separately for each litter and rock shelves sample for 2010 and 2021 (Dzięczkowski 1972) and the dominance classes were determined: D1 – sub-recendents (0–1.0%), D2 – recedents (1.1–5.0%), D3 – subdominants (5.1–10.0%), D4 – dominants (10.1–20.0%), D5 – eudominants (20.1–100.0%). To compare the similarity of gastropod communities between the studied locality and the other areas, the Nei (IN) index was used (Pokryszko & Cameron 2005).
Table 1
List of gastropod species collected in the Bolków castle in particular years. Data from 1843 to 1984 come from publications of Scholtz (1843), Reinhardt (1874), Merkel (1894), Sprick (1921), Pokryszko (1984). In 2010 and 2021, three types of sampling methods were specified: E – manual, by eye, L – litter sample, S – rock shelves and walls sample. Other abbreviations: EG – ecological groups: F – shade-loving species, B – species of partially shaded habitats, M – mesophilous species, O – open-country species, X – xerophilous species (Alexandrowicz & Alexandrowicz 2011); classes of the number of collected individuals of particular species from I to VI: I (1–3), II (4–10), III (11–31), IV (32–62), V (63–99), VI (100–170)

RESULTS
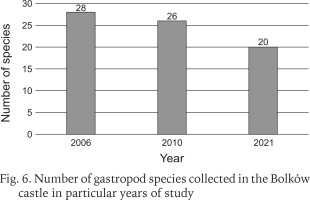
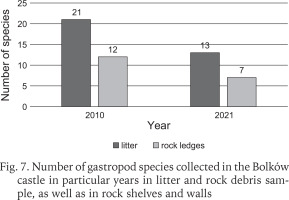
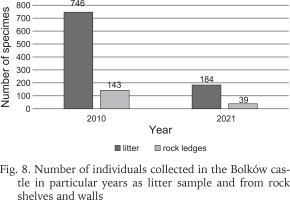
Including the earlier studies (Scholtz 1843, Reinhardt 1874, Merkel 1894, Sprick 1921, Pokryszko 1984), 37 species have been recorded at Bolków castle (Table 1). Two species, Ena montana (Draparnaud, 1801) and Clausilia pumila (C. Pfeiffer, 1828) were found only in older studies before 1984. Our surveys record 35 species, but the number recorded has declined over time, despite the introduction of litter sampling. The decline is most severe between our surveys of 2006 and 2021, at nearly 29% (Fig. 6). Our quantitative samples also show declines in richness and abundance between 2010 and 2021 (Fig. 7). The Chao indices indicate total faunas of 26 species for 2010, and 20 for 2021; the decline is not the result of inadequate sampling.
Fig. 7
Number of gastropod species collected in the Bolków castle in particular years in litter and rock debris sample, as well as in rock shelves and walls
The number of individuals of each species can be counted only for the quantitative samplings, i.e. litter and rock shelves. These values in the form of abundance classes are included in Table 1. The sum of recorded individuals in 2010 and 2021 found in both of the aforementioned samples indicated a drastic decrease in the number of individuals by about 70% in each case (Fig. 8).
Fig. 8
Number of individuals collected in the Bolków castle in particular years as litter sample and from rock shelves and walls
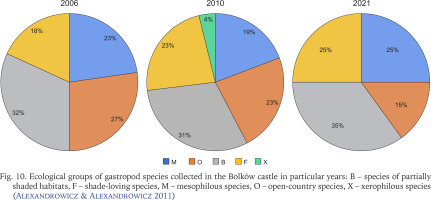
The proportion of species in each ecological group overall (Fig. 9) and in each year of sampling (Fig. 10) shows that shade-loving species are the most frequent. They were represented by 11 species, and together with snails and slugs from partially shaded habitats (B, 9 species) they made up 54% of malacocoenosis (Fig. 9). In individual years, the proportions of ecological groups did not differ much and were more similar in 2010 and 2021 compared to 2006, where species preferring partially shaded habitats and open-country species prevailed in similar proportions (Fig. 10). The most drastic decline in the diversity was among open-country species with values of 18% and 42% between consecutive sampling years. The single xerophilous species, represented by one individual of Cecilioides acicula (O. F. Müller, 1774), was found only in 2010.
Fig. 9
Ecological groups of gastropod species collected in the Bolków castle in all years in total: B – species of partially shaded habitats, F – shade-loving species, M – mesophilous species, O – open-country species, X – xerophilous species (Alexandrowicz & Alexandrowicz 2011)
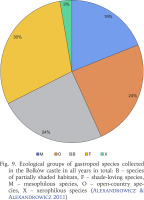
Fig. 10
Ecological groups of gastropod species collected in the Bolków castle in particular years: B – species of partially shaded habitats, F – shade-loving species, M – mesophilous species, O – open-country species, X – xerophilous species (Alexandrowicz & Alexandrowicz 2011)
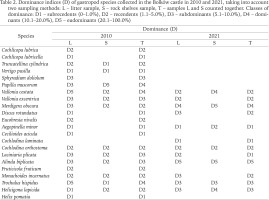
The dominance indices (D), calculated for two sampling methods from 2010 and 2021, and both methods for a given year calculated jointly, are presented in Table 2. The results showed that in the litter sample of 2010, the eudominants (D5) were T. hispidus and V. costata, which comprised 22.25% and 21.05% of the sample, respectively. In the samples from rock shelves and walls from the same year, the undeniable eudominant was P. muscorum (72.03%). This species had disappeared in 2021. No eudominant species was determined when considering the combined calculations for both sampling methods. In 2021 A. biplicata was eudominant, accounting for 54.1% and 35.9% of the samples in the litter and in rock shelves, respectively. It reached 50.9% dominance when both sampling methods were calculated together. Additionally, in the rock shelves M. obscura reached a level of D5 with a dominance value of 20.51% in 2021.
Table 2
Dominance indices (D) of gastropod species collected in the Bolków castle in 2010 and 2021, taking into account two sampling methods: L – litter sample, S – rock shelves sample, T – samples L and S counted together. Classes of dominance: D1 – subrecedents (0–1.0%), D2 – recendents (1.1–5.0%), D3 – subdominants (5.1–10.0%), D4 – dominants (10.1–20.0%), D5 – eudominants (20.1–100.0%)
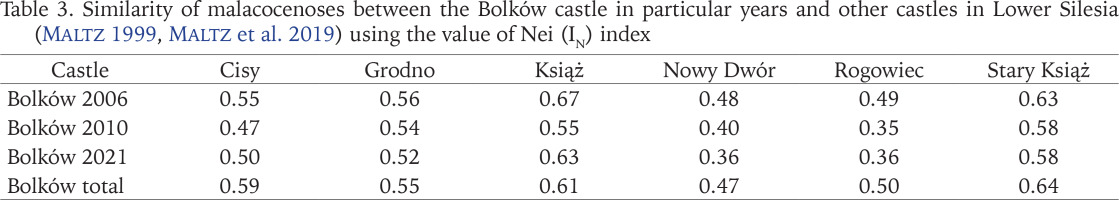
The similarity of malacocoenoses among the Bolków castle and other castles examined in Lower Silesia (based on: Maltz 1999, Maltz et al. 2019) was determined using the Nei (IN) index (Table 3). The most similar composition of the species to Bolków were the castles of Książ and Stary Książ, while the least similar were the Rogowiec and Nowy Dwór castles. The Nei index for Bolków malacocoenosis and the adjacent Kaczawskie Mountains and Foothills (Pokryszko 1984) in 2006 was 0.53, while for 2010 and 2021, it was 0.50. When joint data from Bolków was used, the Nei index was 0.53.
DISCUSSION
Repeated surveys of well-defined sites, using standard methods, are a crucial component of tracking environmental change, whether natural or anthropogenic. They are particularly useful when the sites chosen hold species that are rare or endangered. They can lead to changes in management policies that aid conservation. In this context, castles, and especially those in a ruined state, are of great value, as they present a range of microhabitats wider than that in comparable areas of natural or human-managed places.
The medieval castles of central Europe thus offer an excellent resource for studying faunal change. When ruined, they provide a wealth of varied habitats, albeit ones that change over time. The evidence, presented here and elsewhere (Juřičková 2005), shows that castles also differ in their faunas, reflecting differences in their history, their management after abandonment, and the nature of the surrounding environment. Much of the observed diversity in castle faunas reflects the unusual mixture of shaded and open, often rocky, microhabitats.
On exposed castle towers and parts of the walls uncovered by trees, numerous species of xerothermic plants, mainly succulents and grasses, as well as lithophytes, usually lichens, can occur. Among animals, exposed spaces of walls are inhabited by xerothermic insects, mainly species of Coleoptera, Hymenoptera, Diptera, Hemiptera, Orthoptera and Rhopalocera (Mazur & Kubisz 2000). Places with high insolation also attract representatives of reptiles, including Lacerta agilis, Lacerta vivipara and the Podarcis muralis species recorded in Poland since 2011 (Wirga & Majtyka 2013, Kolenda et al. 2020). Among snails, species such as Pupilla muscorum, Vallonia costata, Truncatellina cylindrica and Euomphalia strigella are mentioned (Alexandrowicz & Alexandrowicz 2011). All have been found at least once in the study of Bolków castle. Other open-country species were present there, for example Cochlicopa lubricella, Vallonia excentrica, V. pulchella, Deroceras agreste and D. reticulatum.
Equally, castles may provide pockets of shaded habitats that are scarce in a predominantly cleared landscape. Bushes and trees grow in a place no longer used. At Bolków, there are a large number of shade-loving species such as Monachoides incarnatus, Cochlodina orthostoma, Merdigera obscura, Sphyradium doliolum and Vertigo pusilla (Alexandrowicz & Alexandrowicz 2011). Furthermore, six other shade-loving gastropods and numerous species of partially shaded habitats were recorded there (Table 1).
The ecological structure of Bolków malacocoenosis is not dominated by a single ecological group. The group of shade-loving species is now slightly more numerous than the others, and the group of mesophilous species is slightly smaller than the rest. However, these differences are not statistically significant, so they cannot indicate any trend. The lack of dominance of any of the groups may be due to the state of preservation of the ruins, where the walls in some parts are completely overgrown with vegetation, while in others exposed to sunlight. Slightly larger differences may be detected by analysing the changes in ecological groups over the years. The increasing presence of shade-loving species and species preferring partially shaded habitats was observed, while the study showed a decrease in the number of open-country species. This trend, due to the insufficient number of repetitions, cannot be confirmed statistically; however, it may become the basis for future studies to learn about changes in the local ecological structure of the malacofauna. We notice that overall abundance has declined, and the renovation of the castle walls in 2015 may be significant. Certainly, open-country species such as Truncatellina cylindrica and Pupilla muscorum appear to have disappeared completely. In addition, the trees surrounding the castle have grown over time, causing more shading. This has created better conditions for shade-loving species, like Merdigera obscura, that increased in abundance. In the hills of the Lipowiec castle, shade-loving species were also numerous, with a rich population of rare Sphyradium doliolum (Alexandrowicz & Alexandrowicz 2017). The opposite trend was in Wawel Hill, where between 1988 and 2011, some open-country species had increased in abundance at the expense of others (Alexandrowicz 1988, Gołas-Siarzewska 2013). In the Smoleń castle, the predominance of gastropods preferring open habitats was also noted. In the period preceding the castle’s construction, the hill was primarily inhabited by mesophilous and shade-loving species, but later anthropogenic activities caused changes in the diversity of malacofauna (Alexandrowicz & Alexandrowicz 2014).
While castles can be seen to provide suitable habitats to support a varied flora and fauna, often including rare species (Alexandrowicz & Alexandrowicz 2014, Wójcik & Ziaja 2015), and, with calcium enrichment, this is particularly the case for molluscs, there are important differences among them. Bolków castle has its own peculiarities when compared to others. The first, very fragmentary data on the molluscan fauna of Bolków castle come from the 19th and early 20th centuries (Scholtz 1843, Reinhardt 1874, Merkel 1894, Sprick 1921) and from a later paper on the malacofauna of the Kaczawskie Mountains and Foothills (Pokryszko 1984). The oldest publications mentioned nine species, including Cochlicopa lubrica, Merdigera obscura, Arion fuscus, Alinda biplicata, Euomphalia strigella, Helicigona lapicida, Discus rotundatus, Clausilia pumila and Ena montana (Table 1). The last two species have not been recorded since. The 35 species recorded this century places Bolków in the mid-range of richness; there were more than 40 at Rogowiec (Maltz et al. 2019), while only 21 were found at Lipowiec (Alexandrowicz & Alexandrowicz 2017).
The malacofauna of Bolków castle included five red-listed species (Wiktor & Riedel 2002), among which were: Sphyradium doliolum, classified as vulnerable (VU), Eucobresia nivalis and Helicigona lapicida as near threatened (NT), and Cecilioides acicula as data deficient (DD).
Sphyradium doliolum is an extremely shade-loving species that lives in damp forests, in leaf litter and between rock rubble (Wiktor 2004). It was only observed in isolated sites in the south of Poland. In the Sudetes, it is known from several localities (Mt. Stary Wapiennik, Mt. Ostrzyca, Mt. Miłek and the Wleń castle) in the Kaczawskie Mountains (Pokryszko 1984), and from the Rogowiec castle ruin (Maltz et al. 2019). It was also found in five Carpathian castles (Alexandrowicz 1995) and in the two castle ruins in Kraków-Częstochowa Upland (Alexandrowicz & Alexandrowicz 2014, 2017). Castles with their isolated and insular populations can be considered local refugia (Alexandrowicz & Alexandrowicz 2017). It appears to have declined at Bolków between 2010 and 2021.
Eucobresia nivalis is considered a mountain species, often sheltering under rocks, logs or in dense vegetation (Wiktor 2004). In Poland, it reaches its northern distribution border, occurring in the Carpathians and a part of the Sudetes, where it is considerably rarer (Umiński 1980). The record in the Bolków castle is the first in the Sudetes Foreland, north of the so far reported occurrence in Ziemia Kłodzka (Umiński 1980). In terms of domination and constancy E. nivalis was a rare species in the ruins of Carpathian castles in Lanckorona, Muszyna and Rytro (Alexandrowicz 1995) as well as in the Lipowiec castle (Alexandrowicz & Alexandrowicz 2017), located in Kraków-Częstochowa Upland. It was absent in 2021.
Helicigona lapicida was observed in seemingly random places in Poland, i.e. in Kraków-Częstochowa Upland, the Sudetes and Western Pomerania. It is abundant in these areas, but only in deciduous forests or limestone rocks. It often hides under stones, in rock crevices or castle ruins (Wiktor 2004). As a “castle species”, it was found in nine Polish castles (Pokryszko 1984, Maltz 1999, Alexandrowicz & Alexandrowicz 2014, Maltz et al. 2019) and 89 Czech castles (Juřičková 2005). It is still present.
The DD status of Cecilioides acicula does not mean that it is a rare in Poland, but rather rarely found due to the subterranean mode of life (Wiktor 2004). As a species tending to colonise anthropogenic habitats, it may have been imported by humans also to castles (Alexandrowicz 1995, 2013, Gołas-Siarzewska 2013). Another noteworthy species is the shade-loving and rock-dwelling Cochlodina orthostoma, known from the southern Sudetes to the Bieszczady Mountains and from several small areas in the north of Poland. These are mainly single habitats in Mazury, Pomerania, the Świętokrzyskie Mountains and the Białowieża Forest (Wiktor 2004). This species also occurs in ruins, however, avoiding restored castles (Alexandrowicz 1995, Maltz 1999, Juřičková & Kučera 2005, Alexandrowicz & Alexandrowicz 2014). Among Bohemian castles, it is the only species that suffered population decline over 50 years (Juřičková & Kučera 2005). It survives at Bolków.
The castle ruins most malacologically similar to Bolków are those of Stary Książ and Książ (Table 3), located a short distance of about 25 km from Bolków. The Cisy castle is even closer to Bolków, 20 km away. Its slightly less similarity may be due to the different nature of the habitats and thus different species composition. The Cisy castle is basically just a ruin, with only sparse fragments of walls left, where 34 species were found (Maltz 1999). The castles of Cisy, Książ and Stary Książ resembled the Bolków castle more than the malacofauna of the neighbouring Kaczawskie Mountains. This may be because of the so-called castle species, which are more frequent or abundant in castles than in natural habitats (Juřičková & Kučera 2005).
While there seems to have been little change in the balance of ecological groups at Bolków, the changes in particular species, and the overall decline in species richness and abundance over the period of surveys are causes of concern. Even with enhanced sampling effort, the fauna recorded in 2010 is marginally smaller than that of 2006, and the drop in richness and abundance between 2010 and 2021 is great, despite the short interval between surveys (11 years). The renovation work may have caused some of these changes, as may have increased recreational use of the castle. The apparent extinction of Pupilla muscorum might be attributed to this, as might the loss of other open-country species such as Cochlicopa lubricella and Truncatellina cylindrica. This ecological group also included Vallonia pulchella, Deroceras agreste and D. reticulatum, which were recorded only in 2006 (Table 1). Repeated surveys of other castles, with different patterns of use and development, would help to determine this.
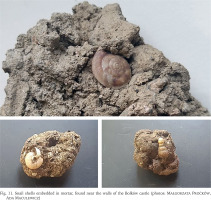
One species running contrary to the general trend is Alinda biplicata, which has increased in abundance. It is one of the so-called castle species (Alexandrowicz 1995, 2013, Juřičková 2005, Alexandrowicz & Alexandrowicz 2014, 2017, Gołas-Siarzewska 2013, Maltz et al. 2019) that with its outstanding colonising abilities (Maltz & Sulikowska-Drozd 2014), it is also a common component of the local malacofauna (Pokryszko 1984, Maltz 1999). It is reassuring that the red-listed (Wiktor & Riedel 2002) Helicigona lapicida has maintained its abundance, a species protected by law (Dziennik Ustaw 2016). Given its accidental inclusion in the mortar used in renovation (Fig. 11), some crevices suitable for resting would have been destroyed. By contrast, some shade-loving species such as Vertigo pusilla and Eucobresia nivalis, have vanished. Again, other surveys would reveal if this is a local or a more widespread change.
Fig. 11
Snail shells embedded in mortar, found near the walls of the Bolków castle (photos: Małgorzata Proćków, Ada Maculewicz)
Our survey can relate at least some faunal change to local factors, not only the renovation, but also the greatly increased use of the castle and its surroundings. This increase will continue, and some restrictions on access might be need to protect fauna and flora. We cannot directly assess the effects of more general changes, such as climate (Korn 2016), nor, indeed the effect of time of year in which samples are made (Wiktor 2004). Our samples were made at different times of year (August in 2006, September in 2010, and late May in 2021).